According to modern concepts, calcium antagonists are a large and rather heterogeneous group of drugs in chemical structure, united by one common property - competitive antagonism of voltage-dependent calcium channels of cell membranes. Calcium antagonists act on L-type, or slow calcium channels, so this group of drugs is more accurately called “slow calcium channel blockers” or “calcium channel blockers” (CCBs). Calcium antagonists have been used in cardiology for more than 30 years. Their widespread use in clinical practice was facilitated by high anti-ischemic and antianginal efficacy, as well as good tolerability, established in large clinical studies. The priority for the discovery of compounds that selectively block the flow of calcium ions directed into the cell belongs to A. Fleckenstein (1964). He was the first to propose in 1969 the term “calcium antagonists” to denote the pharmacological properties of drugs that simultaneously had a coronary vasodilating and negative inotropic effect [22]. The effect of these drugs on the myocardium was very similar to the signs of calcium deficiency described by Ringer in 1882 [33]. The first representative of the BKK, verapamil, was synthesized on May 21, 1959 by Dr. Ferdinand Dengel - this happened 10 years earlier than the term “calcium antagonists” appeared. In 1963, the use of verapamil in the clinic for the treatment of angina pectoris began. In the 70s of the last century, two other representatives of BCC were created and began to be used in the clinic - nifedipine and diltiazem. Since that time, BCC has taken a strong position in cardiological practice. Over the past few years, there has been an active increase in the arsenal of drugs used in this class. The forms of pre-existing drugs are being improved, new chemical compounds are being synthesized, and the indications for their use are being revised.
Mechanism of action of calcium channel blockers: relation to clinical use
The widespread introduction of CCBs into clinical practice has led to a detailed study of calcium homeostasis. It was found that ionized Ca2+ takes part in the regulation of most intracellular processes (automaticity of sinus node cells, contraction and relaxation of the myocardium, incretion, division and growth of cells), and has a connecting role between exogenous factors and regulatory intracellular mechanisms.
The regulation of the physiological response of cells of the cardiovascular system is based on the different permeability of cell membranes to Na, K, and Ca ions. The membrane controls the movement of these ions using ion pumps (for example, for Na, K, etc.), ion exchange (in particular, the exchange of Na for Ca ions) and selective ion channels (for Na, K or Ca ions). The latter open in response to a transmembrane potential difference or when agonists bind to receptors. It has been shown that up to 10 million ions can enter the cell every second through one channel [32]. Calcium ions enter the cytoplasm using all the described mechanisms. However, voltage-gated calcium channels, which open when the cell membrane is depolarized, are responsible for the excitation-contraction process and the main action of BCCs [26]. Calcium channels are macromolecular proteins that “cut” cell membranes. Through these channels, calcium ions move into the myofibril cell and out of the cell.
Calcium channels have the following features: each channel passes about 30,000 calcium ions per 1 s; the selectivity of the channels is relative, since sodium, barium, strontium, and hydrogen ions also enter through them; channel pore diameter 0.3–0.5 nm; the entry of calcium ions through channels after depolarization of the cell membrane occurs more slowly than the entry of sodium ions, therefore voltage-gated calcium channels are called slow, in contrast to fast sodium channels [24]. The function of the channels changes under the influence of various inorganic (cobalt, manganese, nickel ions) and organic inhibitors (drugs - calcium channel inhibitors). There are six types of voltage-gated calcium channels [35]. The most important types in the cardiovascular system are L- and T-types [16]. T- and L-type channels are found in the myocardium and vascular smooth muscle. T channels are quickly inactivated and calcium flow through them is negligible. L channels are inactivated slowly, allowing most of the extracellular calcium to enter the cell. L-channels are sensitive to the action of CCBs; T- and N-channels do not have receptors for calcium antagonists.
L-type calcium channels consist of 5 subunits - alpha-1 and -2, beta, gamma and sigma [17]. The subunit that functions as a calcium channel is of primary importance. Other subunits play a stabilizing role. On the surface of the subunit there are receptors with which CCBs interact.
The current of calcium ions through L-channels forms a plateau of the action potential [27]. In the sinus node (SN), calcium ions take part in providing the pacemaker function; in the atrioventricular (AV) junction they regulate the conduction of excitation [25]. In smooth muscle tissue, L-type channels are necessary for the electromechanical coupling of excitation and contraction processes [10]. Blocking slow BCC channels prevents the entry of Ca2+ ions into the cell and inhibits or completely blocks contraction without significantly affecting the action potential, that is, excitation is decoupled from contraction [15].
In the movement of Ca2+ ions in excitable cells, two cycles are distinguished - extra- and intracellular. As a result of the extracellular cycle, Ca2+ ions enter the cell, bind to the protein troponin and trigger the intracellular calcium cycle, during which Ca2+ ions are released from the sarcoplasmic reticulum, necessary for coupling the processes of excitation and contraction in the heart - calcium-induced calcium release [20, 28]. In smooth muscle fibers (SMCs), contraction begins after calcium binds to calmodulin [27]. In cardiomyocytes, membrane depolarization triggers a rapid "phasic" contraction that correlates with L channel activity. In vascular cells, membrane depolarization is induced by a cascade of intracellular processes following the activation of membrane receptors by hormones and neurotransmitters, which leads to a slowly developing and long-lasting tonic contraction of SMCs [29].
T-type channels are found in vascular SMCs, including coronary, renal and cerebral, but are practically absent in adult SMCs. T channels are detected only with myocardial hypertrophy or proliferation of SMCs in the vascular wall [23, 26]. T-type calcium channels were also found in such excitable tissues as neurosecretory cells innervating vasomotor centers in the brain stem, the cortical and medulla layers of the adrenal glands, and the juxtaglomerular apparatus of the kidneys [24]. Like L-type, T-type channels open when the membrane is depolarized. However, the membrane potential at which T channels open is significantly less than the potential that opens L channels; they are equally permeable to Ca2+ and Ba2+ ions and are quickly inactivated [36]. In smooth muscle, T channels play a role in maintaining vascular tone. In addition, T channels play an important role in the pacemaker activity of the SG and impulse conduction [38]. N-type channels are found only in neuronal membranes.
Cells such as cardiomyocytes and vascular smooth muscle cells have low calcium stores in the sarcoplasmic reticulum and will therefore be particularly sensitive to blockade of transmembrane Ca2+ current.
The amount of calcium and the kinetics of its penetration into the cytosolic space determine the speed and force of contraction of cardiomyocytes, and the kinetics of calcium dissociation with regulatory proteins determine the rate of relaxation in diastole. In therapeutic doses, CCBs do not cause complete blockade of calcium channels, since this is incompatible with life, but only normalize the transmembrane calcium current, which is increased in pathological conditions. It should be noted that each BCC has a “personal” fixation locus [21]. CCBs block the entry of calcium into the cell, reducing the conversion of phosphate-bound energy into mechanical work, and thus reduce the ability of the muscle fiber (myocardial or vascular) to develop mechanical tension. The result of the above is relaxation of the muscle fiber, which causes the appearance of a number of phenomena at the organ level. Thus, the effect of CCB on the wall of the coronary arteries leads to their expansion (vasodilation effect), and the effect on peripheral arteries leads to a decrease in systemic blood pressure (BP) (due to a decrease in peripheral resistance). Overload of cardiomyocytes with calcium ions is largely responsible for mitochondrial damage in ischemic myocardium. A decrease in the amount of calcium supplied to the contractile system leads to a decrease in the breakdown of ATP, energy consumption for contraction and myocardial oxygen demand. Under conditions of ischemia and hypoxia, CCBs, preventing calcium overload, have a protective effect on the myocardium - preventing functional and structural damage to cardiomyocytes. These properties of CCBs reduce the adverse effects of myocardial ischemia and restore the disturbed balance between myocardial oxygen demand and its delivery. Blocking calcium entry into platelets inhibits their aggregation. There is also evidence of the antiatherosclerotic effect of BCC. Other extracardiac effects of CCBs are a decrease in pressure in the pulmonary artery in combination with bronchial dilatation, an effect on cerebral circulation; Antiarrhythmic properties stand somewhat apart.
Thus, the main effects of BCC are the following [4]:
1. CCBs affect the transmembrane entry of Ca through slow channels into cardiomyocytes during excitation. This reduces the Ca-dependent breakdown of ATP, the force of myocardial contraction and the contracting heart's need for oxygen.
2. CCBs reduce the tone of the smooth muscles of the vascular wall, which depends on Ca ions, and eliminate (prevent) their spastic contraction. Dilatation of systemic vessels, mainly arterioles, reduces resistance in the systemic circulation and reduces afterload on the heart.
3. CCBs increase coronary blood flow in ischemic areas by reducing coronary spasm and contraction, as well as by vasodilation of the collateral bed.
4. A decrease in the entry of Ca into the cells of the sinoatrial and atrioventricular nodes slows down the frequency of spontaneous excitations of the normal pacemaker of the heart, as well as the speed of atrioventricular conduction. Most CCBs inhibit ectopic automatism in the area of damaged myocardium.
5. Reduce platelet aggregation and thromboxane formation.
6. Limit lipid peroxidation, which prevents the formation of free radicals.
7. Exhibit antiatherogenic properties; in the early stages of atherosclerosis, they prevent the formation of new atherosclerotic plaques; inhibit stenosis of the coronary arteries, suppressing the proliferation of smooth muscle cells of the vascular wall.
Main therapeutic effects of calcium antagonists
The main properties of calcium antagonists ensure the good functioning of cardiovascular structures and the brain. There is a noticeable decrease in blood pressure. Its widespread use in clinical practice is due to the following therapeutic effects:
- relaxation of smooth muscle vascular muscles;
- decreased blood pressure on the heart valves, ventricles, and myocardium;
- restoration and normalization of cerebral, peripheral or main blood flow;
- normalization of blood circulation in the small circle;
- antihypertensive, vasodilating effect.
At the same time, the automatism of the cellular structures of the sinus node slows down, the risk of the appearance of ectopic foci in the tissues of the heart decreases, and the speed of impulse delivery through the atrioventricular node decreases.
Their use along with other antihypertensive drugs enhances the effect of BMCC, improves vascular conductivity and myocardial contractility. The classic regimen of use looks like this: blockers + ACE inhibitors + diuretics and auxiliary drugs. A similar scheme is applicable for chronic heart failure, cardiomyopathy, stenosis of blood vessels and arteries.
Classification of calcium channel blockers
In 1987, the WHO Expert Committee divided CCBs into two groups - selective and non-selective, identifying 6 classes among them depending on the chemical structure.
The following three classes are classified as selective BCCs
1. Phenylalkylamines (verapamil and its derivatives).
2. Dihydropyridines (nifedipine and its derivatives).
3. Benzothiazepines (diltiazem and its derivatives).
Tissue selectivity in the action of these classes of BCC is manifested in the fact that they do not act on skeletal muscles, muscles of the bronchi, trachea and intestines, as well as on nervous tissue. Therefore, they are not characterized by the development of corresponding adverse reactions and a negative impact on the quality of life. This compares them favorably with β-blockers [12, 13].
In 1996, T. Toyo-Oka and W. Nayler recommended a classification of CCBs, which reflected the evolution of the creation of these drugs (Table 1). This classification is based on the following: 1) the chemical structure on which the pharmacological effects of the drug depend. For example, dihydropyridines have a greater effect on vascular smooth muscle and have virtually no effect on the myocardium and conduction system of the heart. Phenylalkylamines (verapamil), on the contrary, have a greater effect on the myocardium, the functions of the sinus and atrioventricular nodes than on vascular smooth muscle; 2) pharmacokinetics.
Long-acting dosage forms of calcium antagonists are divided into two subgroups: subgroup IIa includes drugs whose effect is prolonged by placing the drug in a special tablet or capsule that provides delayed release of the drug. Subgroup IIb includes drugs whose effect is prolonged due to the ability to circulate in the blood for a longer period of time [19].
In 1997 B.A. Sidorenko et al. [14] recommended a modern classification of CCBs, which took into account the ability of these drugs to influence L-, T- and R-types of calcium channels and the presence of additional properties (Table 2).
The classification of CCBs is very important for the clinician, dividing all drugs into two large subgroups based on their effect on the tone of the sympathetic nervous system. The first subgroup is the so-called pulse-slowing calcium antagonists (or non-dihydropyridine calcium antagonists). These actually include two drugs - verapamil and diltiazem. The second subgroup is pulse-increasing calcium antagonists, or dihydropyridines [1].
Pharmacological interaction
Before starting a course of taking non-dihydropyridine blockers simultaneously with other medications, it is necessary to check the drug combination.
- Phenylalkylamine calcium antagonists should not be taken concomitantly with selective blockers.
- Antihypertensive blockers should not be taken with Procainamide and anticonvulsants.
- Taking calcium channel blockers with NSAIDs and sulfonamides may lead to increased adverse reactions from taking CCBs.
- Taking calcium antagonists is allowed together with angiotensin-converting enzyme inhibitors, nitrates, and diuretics.
General characteristics of calcium channel blockers
First generation calcium channel blockers include nifedipine, verapamil and diltiazem. All these drugs were obtained in the 60s of the twentieth century and retain their importance to this day (they are called first generation drugs or prototype drugs). The three main drugs in this group differ significantly in chemical structure, binding sites on calcium channels, and tissue vascular specificity.
Thus, the selectivity of the dihydropyridine CCBs nifedipine and amlodipine for blood vessels is 10 times higher, for felodipine and isradipine – 100 times, and nisoldipine – 1000 times higher for the myocardium, compared with verapamil and diltiazem [7]. Dihydropyridine CCBs have a less pronounced cardiodepressive effect and do not affect the sinus and AV node. Reduced afterload and coronary vasodilation are most pronounced in this group. Short-acting nifedipine is used today mainly for the relief of hypertensive crises, while other long-acting forms of nifedipine, among other CCBs, are recommended for long-term treatment of patients with coronary artery disease and arterial hypertension.
Derivatives of diphenylalkylamine (verapamil group) and benzothiazepine (diltiazem group) have an effect on both blood vessels and the heart. They inhibit the automatism of the sinus node, lengthen atrioventricular conduction, increase the refractoriness of the atrioventricular connection, reduce myocardial contractility, reduce peripheral vascular resistance and prevent spasm of the coronary arteries. Drugs of these groups reduce heart rate; verapamil has a more characteristic negative inotropic effect [9]. Non-dihydropyridine CCBs are described as having a frequency-dependent effect: the more frequently calcium channels open, the better the penetration of non-dihydropyridine CCBs to binding sites [10]. This explains their effect on the tissues of the AV node during paroxysmal tachycardias. Thus, CCBs of the verapamil and diltiazem groups have antianginal, antiarrhythmic and hypotensive effects.
However, the short duration of action of the prototype drugs required repeated administration throughout the day, which created certain inconvenience for patients. Taking short-acting CCBs was accompanied by a large range of therapeutic drug concentrations in plasma, causing “peaks” and “troughs”, which led to instability of the vasodilating effect and was accompanied by reflex neurohumoral activation. As a result, there was an increase in blood pressure variability (fluctuations) and heart rate, and the daily blood pressure curve resembled the teeth of a saw.
These points deserve special attention, since tachycardia and blood pressure variability are independent risk factors for the progression of complications of arterial hypertension. In addition, when using first-generation CCBs in elderly patients, their direct negative inotropic effect may occur with subsequent inhibition of myocardial function [12].
Probably, these circumstances led to the search for the possibility of creating prototype drugs with prolonged action, which could lead to a single, or maximum twice, dose of the drug. This desire led to the creation in the 80s of the 20th century of calcium antagonists of the second generation, which have a longer duration of action, good tolerability, tissue specificity, and selectivity.
Today, in the group of CCBs - dihydropyridine derivatives - modern prolonged dosage forms have almost completely replaced short-acting first generation drugs.
New generation drugs come in various dosage forms: - slow-release - retard or slow-release (in the form of tablets and capsules); - with two-phase release (rapid-retard); — 24-hour therapeutic systems (GITS system).
Second-generation CCBs have an improved pharmacokinetic profile and higher vasoselectivity. Compared to first generation drugs, they are characterized by a longer half-life (for first generation CCBs T1/2 is 4–6 hours, second generation — 12–24 hours), a longer duration of action, a smoother increase in drug concentration in the blood plasma (no peak-shaped changes in concentration), delayed onset of action and time of peak effect. In practical terms, this determines the fact that second-generation CCBs are devoid of many side effects of first-generation drugs, primarily associated with reflex activation of the sympathoadrenal system, and also have a dosage regimen that is more convenient for the patient (1-2 times a day). Long-acting nifedipine preparations dilate the main coronary arteries and arterioles (including in ischemic areas of the myocardium) and prevent the development of coronary artery spasm. Thus, nifedipine preparations improve the supply of oxygen to the myocardium while reducing the need for it, which allows their use in the treatment of angina pectoris [9, 32]. Pronounced vasodilation while taking nifedipine is due not only to the blockade of calcium channels, but also to stimulation of the release of nitric oxide by endothelial cells, which is a powerful natural vasodilator; it is also associated with increased bradykinin release [21].
Some new CCBs have better properties than prototype drugs. Thus, gallapamil has a longer action than verapamil. The benzothiazepine derivative klentiazem is 4 times stronger than diltiazem and its antianginal effect is longer lasting. More pronounced vasoselectivity was found among 1-4-dihydropyridine derivatives (felodipine → amlodipine → nifedipine). The drug nimodipine has a higher sensitivity to the cerebral arteries, nisoldipine to the coronary arteries, felodipine has the same effect on the coronary vessels and peripheral arteries. Among CCBs with a different chemical structure, monatepil deserves attention, since this drug has the properties of an α1-adrenergic blocker with pronounced vasodilating effects and a clear hypolipidemic and anti-sclerotic effect. The positive characteristics of second-generation CCBs also include new additional properties, for example, antiplatelet activity against platelets (trapidil).
However, the pharmacokinetic and pharmacodynamic characteristics of second-generation CCBs were still far from ideal. For some drugs there were problems with high bioavailability. The history of the clinical introduction of CCBs was somewhat overshadowed by the experience of using mibefradil, a representative of a new subgroup of selective T-channel blockers, which, having high antihypertensive activity, was withdrawn from clinical use due to numerous cases of interaction with other drugs.
Despite the fact that the advent of second-generation CCBs is associated with undoubted progress in effectiveness and safety, the urgent problem was the creation of more advanced drugs. The requirement for third-generation BCCs was the uniform release of the active substance against the background of a uniformly expressed (including early morning hours) and longer-lasting action. When developing new drugs, the task was to improve organ-protective characteristics, as well as safety in high-risk groups and when interacting with other widely used drugs.
Currently, the third generation BCC group includes three drugs from the group of dihydropyridine derivatives - amlodipine, lacidipine and lercanidipine (Table 1). They differ from other members of the class in their unique way of interacting with high-affinity specific binding sites in calcium channel complexes and in their long duration of action. The vast majority of researchers consider amlodipine to be the reference drug for third-generation dihydropyridine CCBs, which is highly effective, has minimal side effects for its class, and has a very long-lasting effect (for more than 24 hours).
The above characteristics of third-generation CCBs determine a gradual onset and long-term antihypertensive effect. These properties are essential characteristics as they are considered essential for optimal antihypertensive therapy. The experience of clinical use has confirmed the high significance of the ratio (coefficient) of the residual effect to the maximum, as well as minor fluctuations in blood pressure with a single (during 24 hours) administration of the drugs.
The only levorotatory isomer of amlodipine, S-amlodipine, has been synthesized, which retains all the positive effects on the cardiovascular system of the racemic form of amlodipine, but in a very small number of cases causes adverse side effects in the form of peripheral edema. In addition, there is another positive effect of the drug in the form of a lower metabolic load on the liver, because there is no need to metabolize the unnecessary R-isomer. The new form of the drug was shown to have high clinical efficacy at half the dose compared to the racemic form of amlodipine.
In addition to synthetic CCBs, herbal preparations are used. Thus, tetrandrine and tranminone have been used for a long time in Chinese folk medicine to treat coronary insufficiency.
Time to take antihypertensive drugs
The optimal treatment regimen for patients with hypertension is a single dose of antihypertensive drugs with a priority of combination therapy, which ensures blood pressure control for 24 hours, including by increasing treatment adherence. However, the question remains about the best time to take drugs - in the morning or at night. A multicenter, prospective, open-label study in 40 Spanish centers including 19,168 patients with hypertension and various comorbidities (obesity in 30%, diabetes in 24%, CKD in 29%, history of cardiovascular events in 10% of patients) showed the benefits of evening antihypertensive therapy. [thirty]. According to the protocol, patients took 1 or more antihypertensive medications either before going to bed or shortly after waking up. On average, 53% of patients received ARBs, ACE inhibitors - 24%, CCBs - 43%, diuretics - 43%, BBs - 20%. Noteworthy is the high frequency of use of CCBs, which were significantly more often prescribed. The most frequently prescribed monotherapy in both treatment regimens were ARBs or ACE inhibitors and CCBs (mainly amlodipine, 13% of patients). Participants took an average of 1.8 antihypertensive medications per patient [30].
During follow-up (median 6.3 years), patients taking medications at bedtime had a significantly lower rate of primary endpoint events (cardiovascular death, MI, coronary revascularization, heart failure, or stroke) compared with those taking in the morning. by 45% [0.55 (95% CI 0.50–0.61), p<0.001] and each of its individual components (p<0.001 in all cases), i.e. the probability of cardiovascular death decreased by 56% [0.44 (0.34-0.56)], MI - by 34% [0.66 (0.52-0.84)], coronary revascularization - by 40% [0.60 (0. 47–0.75)], heart failure – by 42% [0.58 (0.49–0.70)], stroke – by 49% [0.51 (0.41–0.63)] (Fig. . 4). This group showed improved renal function and a more favorable lipid profile. Thus, in a large-scale comparative study of real-life clinical practice, it was shown that the administration of antihypertensive drugs before bedtime is safe and reduces the risk of cardiovascular complications to a greater extent compared with morning administration [30].
Pharmacokinetics and pharmacodynamics of calcium channel blockers
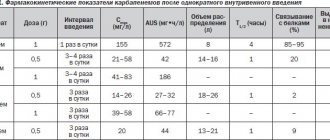
CCBs actively bind to proteins and are subject to the first-pass effect to varying degrees. Their bioavailability varies widely - from 20 to 90% [2]. Data on the pharmacokinetics of CCBs of various groups are given in table. 3.
New dosage forms (Table 4) provide a constant concentration of the drug in the blood and a long-lasting effect. The pharmacokinetics of some CCBs depends on the age of the patients and concomitant pathology. The clearance of nifedipine, verapamil, diltiazem, amlodipine and felodipine may decrease in older age groups [5, 6].
The main pharmacological effects of CCBs are presented in Table. 5. All CCBs reduce afterload. The decrease in systemic vascular resistance and mean aortic pressure after taking nifedipine is significantly more pronounced than after verapamil and diltiazem, and is accompanied by a significant increase in heart rate. According to the degree of afterload reduction, the ejection fraction, cardiac and stroke index of the left ventricle increase.
CCBs clearly improve left ventricular diastolic function, especially associated with myocardial ischemia [8]. There are several hypotheses explaining the prevention of left ventricular dysfunction in patients with coronary artery disease under the influence of verapamil and diltiazem. These CCBs may reduce the prevalence of chronic myocardial ischemia by increasing coronary blood flow through a direct effect on vascular smooth muscle or by enhancing collateral blood flow. This mechanism is most likely in those patients in whom ischemia is more associated with vasoconstrictor vascular reactions than with fixed obstruction.
Another mechanism for preventing ischemic left ventricular dysfunction is to improve the relaxation phase by reducing afterload. This leads to a decrease in myocardial tension and a decrease in its need for oxygen. Finally, the direct effect of CCBs in improving diastolic function may be achieved by suppressing myocardial contractility and thus preserving ATP in the heart muscle. The decrease in ATP demand directly correlates with a decrease in calcium current through slow channels in the early stages of myocardial ischemia. This possibility is assumed only for verapamil and diltiazem, since nifedipine increases myocardial contractility [35].
Consequently, CCBs have a cardioprotective effect, which is realized through improving myocardial perfusion, reducing myocardial oxygen demand, reducing the formation of free radicals and overload of cardiomyocytes with calcium ions, which leads to regression of hypertrophy and clinical damage to the left ventricular myocardium.
How to take it correctly
The order of administration, dosage, and duration of therapy are determined by the attending physician after examination, establishing the exact picture of the disease according to indications.
General recommendations for taking calcium blockers:
| Name | Recommended dose | Frequency of reception |
| Amlodipine | 2.5 mg gradually increased to 10 mg | 1 time per day |
| Isradipin | 2.5 mg gradually increased to 10 mg | 1 time per day |
| Felodipin | 2.5 mg gradually increased to 10 mg | 2 times a day |
| Nifedipine | 5-10 mg | 4 times a day |
| Verapamil | 40-120 mg | 1 per day |
| Gallopamil | 50 mg | 4 times a day |
Doctors recommend increasing the dosage gradually. Increasing the dosage is permissible up to the maximum values specified in the instructions. When a therapeutic effect is achieved, there is no need to increase the dose. Medicines are prescribed by the attending physician according to indications after examination.
Any medications for hypertension can only be taken as prescribed by a doctor.
Effects of calcium channel blockers on renovascular hemodynamics
CCBs have a positive effect on renal hemodynamics. They improve renal circulation, despite the decrease in perfusion pressure as a result of normalization of blood pressure. This effect is realized through a direct effect on vascular tone and an indirect effect - blocking the vasoconstrictor effect of endothelin-1 and angiotensin II. CCBs improve natriuresis and practically do not change the levels of K– and Mg2+ in blood plasma [31].
Some BCCs have an antisclerotic effect on the renal parenchyma. This effect is realized by inhibiting the influence of growth factors and reducing the proliferation of fibroblasts. Among the CCBs, lercanidipine, felodipine and diltiazem have an antisclerotic effect. Long-term use of CCBs (especially lercanidipine and diltiazem) reduces proteinuria. Thus, the DIAL study demonstrated the ability of lercanidipine to reduce albuminuria, which has not yet been proven for other dihydropyridine derivatives.
In recent years, it has been established that long-acting benzothiazepine derivatives exhibit a greater antiproteinuric effect than dihydropyridine derivatives. Diltiazem has been used successfully in dialysis patients, especially in combination with an ACE inhibitor. The use of diltiazem may prolong the survival of the transplanted kidney [35].
Metabolic and pleiotropic properties of calcium channel blockers
CCBs are metabolically neutral, which is manifested by the absence of an effect on purine, carbohydrate, lipid and electrolyte metabolism. In addition, CCBs have other positive supportive effects [22]. They improve the rheological properties of blood, reduce platelet aggregation, and inhibit the progression of atherosclerosis by improving endothelial dysfunction (reducing the effect of endothelin-1 and improving endothelium-dependent relaxation).
Indications for the prescription of calcium channel blockers in cardiological practice
More often, these drugs are used in the treatment of arterial hypertension and coronary heart disease. CCBs other than amlodipine, lercanidipine and felodipine are not used in the treatment of heart failure because they have a negative effect on the inotropic function of the heart. Data have been obtained confirming the possibility of using small doses of diltiazem in patients with dilated cardiomyopathy with an ejection fraction of 50%, that is, in cases of left ventricular diastolic dysfunction. But CCBs are not widely used in the treatment of heart failure.
The combination of coronary artery disease or hypertension with diabetes also brings CCB to the forefront when determining priority areas of modern therapy [3]. The choice of CCBs as a first-line drug in this situation is determined by the presence of the majority of registered indications for the use of these drugs in patients with diabetes: middle and older age groups, isolated systolic hypertension, dyslipidemia, damage to the renal parenchyma, obstructive peripheral circulatory disorders. Finally, the use of CCBs in this combination avoids polypharmacy and increases patient adherence to treatment.
In the treatment of IHD, it is recommended to use non-dihydropyridine CCBs and third-generation dihydropyridines [9]. However, non-dihydropyridine derivatives (verapamil, diltiazem) have an insufficient duration of action and pharmacodynamics are not always predictable. In addition, dihydropyridine derivatives are significantly superior to verapamil and diltiazem in their ability to dilate coronary vessels. In addition, they have virtually no effect on the vegetative status and are metabolically neutral, which gives them undoubted advantages when choosing a drug for patients with diabetes [8].
In patients with supraventricular tachycardias (AV nodal reciprocal, orthodromic tachycardias), verapamil and diltiazem are the drugs of choice for stopping paroxysms (90% effective). In patients with atrial fibrillation and flutter, these CCBs affect AV conduction and reduce heart rate, which has a positive effect on cardiac hemodynamics.
BCBs are perfectly combined with ACE inhibitors, diuretics, nitrates, sartans, and β-blockers (dihydropyridine). Therefore, for the treatment of patients with hypertension and coronary artery disease, these drugs are widely used in combination therapy, which is increasingly used in medical practice.
When combining CCBs with other drugs, it must be remembered that verapamil increases the concentration of digoxin by 50–70%, while other CCBs do not affect the pharmacokinetics of cardiac glycosides. Verapamil (to a lesser extent diltiazem) in combination with β-blockers has a synergistic effect on myocardial contractility, cardiac conduction system and sinus node function. In addition, it must be remembered that verapamil in combination with disopyramide enhances the negative inotropic effect characteristic of these drugs, so this combination is considered dangerous. The proarrhythmogenic properties of antiarrhythmic drugs are also enhanced [38].
In what cases should a doctor prescribe calcium channel blockers?
CCBs are prescribed: - for monotherapy or combination therapy of arterial hypertension; - isolated systolic hypertension in the elderly; - hypertension and the presence of concomitant conditions (diabetes mellitus, bronchial asthma, kidney disease, gout, dyslipoproteinemia); — IHD: stable angina pectoris, vasospastic angina; — IHD with supraventricular rhythm disturbances; — MI without Q wave (diltiazem); — IHD in the presence of concomitant conditions (diabetes mellitus, bronchial asthma, gout, gastric ulcer, dyslipoproteinemia); — IHD in combination with arterial hypertension; - relief of paroxysms of supraventricular tachycardias (tachycardias with a narrow QRS complex < 0.12 s) - verapamil, diltiazem; - reduction in heart rate during paroxysms of atrial fibrillation and atrial flutter (verapamil, diltiazem); - presence of contraindications or poor tolerance to β-blockers - CCBs as an alternative therapy.
What are the possible side effects of calcium channel blockers?
The incidence of side effects (Table 6) is highest with nifedipine (approximately 20%) and significantly less with diltiazem and verapamil (5–8% of patients).
Of the entire group of side effects when taking CCBs, one should especially highlight the appearance of swelling of the ankles and lower legs (this symptomatology is more pronounced if the patient is elderly, has been in an upright position for a long time, has had any injuries to the lower extremities, or has venous pathology). This side effect is difficult to tolerate by patients, which may cause a reduction in the dose of the drug, and in some cases, cessation of effective antihypertensive treatment (9.3% of patients). The withdrawal of antihypertensive therapy is subsequently manifested by an increase in morbidity and mortality in patients with cardiovascular diseases.
Another side effect of CCBs (it concerns mainly drugs of the dihydropyridine group and is associated with their vasodilating properties) is the development of tachycardia and a sudden feeling of heat and flushing of the skin of the face and upper part of the shoulder girdle (so-called flashing).
Side effects of non-selective, or rhythm-slowing, CCBs (verapamil and diltiazem) manifest themselves in the form of a slight decrease in myocardial contractile function, slower heart rate and AV conduction. Even vasoselective dihydropyridine CCBs (eg, nifedipine, amlodipine, and felodipine) may cause some cardiac depression, but this is offset by sympathetic activation of the heart with a slight increase in heart rate that resolves over time.
Contraindications to the use of calcium channel blockers
Absolute: pregnancy (first trimester) and breastfeeding, arterial hypotension (SBP below 90 mm Hg), acute myocardial infarction (first 1–2 weeks), left ventricular systolic dysfunction (clinical and radiological signs of pulmonary congestion, ejection fraction left ventricle less than 35–40%), severe aortic stenosis, sick sinus syndrome, grade II–III AV block, atrial fibrillation in WPW syndrome with anterograde conduction along additional pathways, hemorrhagic stroke in patients with suspected hemostatic impairment.
Relative: 1) for the verapamil and diltiazem groups - pregnancy (late stages), liver cirrhosis, sinus bradycardia (less than 50 beats/min), combination with β-blockers (especially with intravenous administration), amiodarone, quinidine, disopyramide, etacizine , propafenone, prazosin, magnesium sulfate, etc.; 2) dihydropyridine - pregnancy (late stages), liver cirrhosis, unstable angina, hypertrophic cardiomyopathy with severe obstruction, combination with prazosin, nitrates, magnesium sulfate, etc.