Release form: Capsules, No. 14; No. 28.
A probiotic medicinal product containing spores of immunomodulating bacteria, folic acid and cyanocobalamin, to restore one’s own beneficial microflora and intestinal mucosa during dysbacteriosis, due to taking antibiotics and other reasons, and to strengthen the immune system in adults and children.
Manufacturer: Mepro Pharmaceuticals Private Limited, India/Mepro Pharmaceuticals Pvt. Ltd, India; Windlas Healthcare Pvt., Ltd. India/Windlas Healthcare Pvt. Ltd, India.
Registration Certificate: No.UA/0160/01/01 Order of the Ministry of Health of Ukraine No. 1066 dated 12/09/2013.
Go to product website
Read Instructions
Find it in a pharmacy
Pharmacological properties.
Pharmacodynamics. Lactovit Forte is a combination drug that contains Lactic Acid Bacillus (Bacillus coagulans (Lb.sporogenes)), folic acid and vitamin B12.
The therapeutic effect of Lactovit forte is determined by bacteria that produce lactic acid and have antagonistic activity against pathogenic and opportunistic microorganisms. Bacterial spores are activated in the stomach, then in the duodenum they transform into a living vegetative form and create favorable conditions for the development of beneficial intestinal microflora due to the production of L (+) lactic acid. Lactobacilli activate the enzymatic breakdown of proteins, fats and complex carbohydrates (also in cases of lactase deficiency in children) and contribute to the restoration of the intestinal mucosa.
Folic acid (vitamin B9) is necessary for normal leuko- and erythropoiesis, the synthesis of amino acids and nucleic acids, purines and pyrimidines. During pregnancy, it protects the body from the action of teratogenic factors.
Vitamin B12 (cyanocobalamin) is involved in the metabolism of carbohydrates, proteins and lipids, in the synthesis of nucleic acids, helps normalize impaired functions of the nervous system, and stimulates erythropoiesis.
Pharmacokinetics.
Not researched.
Analogs
Level 4 ATC code matches: Bioflor
Enterogermina
Analogues of the drug are:
- Lacidophilus - contains only lactobacilli (Lactobacillus acidophilus and Lactobacillus rhamnosus);
- Linek c - in addition to Lactobacillus acidophilus, the composition includes Bifidobacterium infantis and Enterococcus faecium (combined probiotic).
Indications for use.
Lactovit Forte is prescribed for the treatment of adults and children over 6 months of age who suffer from chronic colitis of various etiologies, including nonspecific ulcerative colitis; in the case of somatic diseases complicated by dysbacteriosis that arose as a result of the use of antibiotics, sulfonamide drugs and other reasons; for the treatment of patients with intestinal disorders who have suffered acute intestinal infections caused by pathogenic and opportunistic bacteria, they are also used in obstetric and gynecological practice for nonspecific inflammatory diseases of the genitals and prenatal preparation of pregnant women at risk with impaired purity of vaginal secretions up to III-IV degrees.
When conducting maintenance therapy for urticaria, eczema, childhood diathesis, atopic dermatitis.
Price Laktovit Forte, where to buy
You can buy it in Moscow pharmacies, which are presented on the website of the Federal Information Service www.poisklekarstv.ru. Information is updated daily. The average price of Laktovit Forte is 1350 rubles. If you are interested in Laktovit tea, the price for 20 bags is 300 rubles.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
LuxPharma* special offer
- Lactovit forte capsules N28
RUR 1,870 order
show more
Pharmacy24
- Laktovit forte No. 28 capsules Mepro Pharmasyut.PVT.LTD..India/Windlas Healthcare Pvt.Ltd..India
149 UAH.order - Laktovit forte No. 14 capsules Mepro Pharmasyut.PVT.LTD..India/Windlas Healthcare Pvt.Ltd..India
78 UAH order
Method of administration and dose.
For colitis, dysbacteriosis, and intestinal disorders, use the drug for adults and children over 6 months 2 times a day 40 minutes before meals or immediately before feeding.
For use in children under 2 years of age, open the capsule and dissolve the contents in 10 ml of breast milk or boiled water at room temperature (the water should not be hot) until a suspension is formed; single dose - 5 ml of suspension.
For children over 2 years of age and adults, the single dose is 1 capsule, the daily dose is 2 capsules. For adults, the maximum single dose is 2 capsules, the maximum daily dose is 4 capsules.
In obstetric and gynecological practice, Lactovit Forte is taken orally in normal doses. For prenatal preparation of pregnant women at risk, the drug is prescribed 2 capsules 1 time per day for 5-8 days.
Newspaper "News of Medicine and Pharmacy" 13-14 (376-377) 2011
Diarrhea that occurs during the use of antibacterial agents and has no other obvious causes is defined as antibiotic-associated diarrhea (AAD). The criteria for this syndrome are loose or pasty stools with a frequency of more than 3 times a day and/or the daily amount of feces exceeding 200 g. AAD can develop in the period from 2 hours after taking an antibiotic to 8 weeks after its discontinuation [2, 8, 28] . It should be noted that in healthy children in the first months of life who are breastfed, the frequency of stool can be from 6 times a week to 10 times a day, and the stool can be soft, mushy or watery and contain some visible mucus [3] . Apparently, any change in the nature of the stool (thinning, the appearance of more mucus) and an increase in the frequency of bowel movements in a newborn compared to the usual nature of bowel movements for a given child can be regarded as diarrhea.
The incidence of AAD among children most depends on the type of antibacterial agent used and varies between 8–30% [5, 21]. The idiopathic form is the leader in the morbidity structure, accounting for 80–90%. The remaining 10–20% of all diarrhea caused by antibiotic therapy are associated with C. difficile [7]. Although only 10–20% of patients with AAD have toxigenic strains of C. difficile, recent attention has focused on this microorganism. This is primarily due to the fact that this microorganism is capable of causing serious clinical manifestations in the form of pseudomembranous colitis, which ends in death in 30–40% of cases [11, 19, 21]. Among newborns, the carriage rate reaches 50–65% and decreases with age [5, 23]. To date, there is no data in Ukraine regarding the incidence of AAD in newborns.
The following risk factors for the development of AAD are identified [1, 6, 8, 10]: long hospital stay; being in a room with other patients infected with C.difficile; treatment in intensive care units; age up to 3 years; old age (over 65); previous surgical interventions; tube feeding; state of immunosuppression; the presence of concomitant anemia, body weight deficiency; use of antacids; severe concomitant pathology (nonspecific ulcerative colitis, Crohn's disease, renal failure, etc.). A positive relationship between the incidence of diarrhea and the route of administration and duration of antibacterial therapy has not been proven. A number of studies have shown that severe forms of AAD can develop even after a single dose of an antibiotic [20].
The spectrum of clinical symptoms can vary from asymptomatic bacterial carriage (especially in newborns and children of the first year of life) or a mild course in the form of watery diarrhea up to 5-7 times a day, not accompanied by systemic manifestations, to severe - diarrhea up to 20 times a day (sometimes with admixture of blood), accompanied by fever up to 39–40 ° C, and the development of severe dehydration. A severe variant is pseudomembranous colitis, which is observed in 1–2% of patients with diarrhea caused by C. difficile infection. The absence of clinically manifest forms of AAD associated with Clostridium difficile in children is apparently explained by the absence or immaturity of receptors of intestinal mucosal cells for the toxins of this pathogen. It is believed that temporary resistance to this infection may also be associated with the presence in children of the first six months of life of maternal anti-clostridial antibodies obtained transplacentally [28].
Probiotics (eubiotics) are a pharmacological group of drugs containing strains of one or more types of microorganisms that have a beneficial effect on the human body. These agents are increasingly used to treat a wide range of gastrointestinal diseases in both adults and children [14, 17, 18]. Currently, sufficient experience has been accumulated in the use of probiotics for AAD. The most studied in this regard are the following microorganisms: Saccharomyces boulardii, bacteria of the genus Lactobacillus (L.rhamnosus strain GG, L.casei, L.acidophilus), Bifidobacterium (B.longum, B.lactis). Probiotics can be used both as a means of preventing diarrhea and for therapeutic purposes. Bacteria of the genus Lactobacillus (L. rhamnosus strain GG, L. casei, L. acidophilus) have been successfully used to treat patients with diarrhea associated with C. difficile. Supplementation with these probiotics resulted in a significant reduction in clinical symptoms of AAD [15, 27, 32, 33]. Recent meta-analyses have shown that probiotics containing Lactobacillus strain GG, L. sporogenes and Saccharomyces boulardii have a significant preventive effect against the development of AAD [22, 29, 30]. S.boulardii reduced the incidence of antibiotic-associated diarrhea by 61%, and lactobacilli by 66% [17, 26, 30, 31]. The use of Bifidobacterium lactis in combination with S. thermophilus for prophylactic purposes prevented the development of AAD in infants [4].
The mechanism of action of these probiotics in the treatment and prevention of AAD is as follows: S. boulardii produces a proteolytic enzyme that prevents the binding of toxins A and B produced by C. difficile to receptors [24]; microorganisms of the genus Lactobacillus stimulate local immunity of the intestinal mucosa (synthesis of IgA and IgG, release of interferon), produce compounds that have antimicrobial activity and prevent the adhesion of enteropathogens to epithelial cells [17]. There is also evidence of the effectiveness of the use of probiotics for the treatment of diarrhea, which is directly related to the damaging effects of antibiotics. It was shown that the use of yogurt containing Bifidobacterium longum led to a reduction in stool frequency in erythromycin-induced diarrhea. In another study, the use of probiotics containing Lactobacillus bacteria reduced the symptoms of ampicillin-induced diarrhea [32].
The effectiveness of probiotics largely depends on properties such as survival and the ability to multiply and colonize in the large intestine. The above microorganisms that are part of probiotics fully possess the indicated properties [9, 16]. Also, numerous studies have shown the absence of serious side effects when using probiotics [11, 12, 25].
Of particular interest is the probiotic Lactovit forte , which contains spores of the bacteria L.sporogenes (according to the new nomenclature B.coagulans). B. coagulans is a Gram-positive, spore-forming, motile bacterium. Spores of this bacterium germinate in the acidic contents of the stomach and penetrate the intestines, where they produce the beneficial L(+) form of lactic acid and effectively prevent the growth of pathogenic microorganisms. Lactic acid produced by B. coagulans creates an optimal acidity of the environment for the development of normal intestinal microflora, has an antibacterial effect, and is also an energy substrate for epithelial cells of the gastrointestinal mucosa. B. coagulans bacteria do not colonize the intestinal mucosa and are gradually eliminated from the gastrointestinal tract, providing a prolonged therapeutic effect after the end of their administration.
A study was conducted with the drug Lactovit forte.
The purpose of the study was to study the effectiveness and safety of using the drug Laktovit forte for the prevention of antibiotic-associated diarrhea in newborns.
Research objectives:
— to establish the incidence of AAD in newborns;
— determine the most significant risk factors for the development of AAD in newborns;
— to evaluate the therapeutic and prophylactic effectiveness and safety of the drug Laktovit forte against diarrhea in newborns associated with taking antibiotics.
The study was conducted at the Odessa Regional Children's Clinical Hospital in the neonatal pathology department.
Materials and methods
The observation group included 60 children from 3 to 28 days of life.
Inclusion criteria: newborn children aged 3 to 28 days inclusive; children with intrauterine infection and other conditions who received parenteral antibacterial therapy (aminopenicillins, cephalosporins, macrolides), at an age-specific dosage of at least 7 days; gender (boys and girls).
Exclusion criteria: lack of informed parental consent; acute gastroenteritis, necrotizing enterocolitis, malformations of the gastrointestinal tract, primary immunodeficiency, malabsorption syndrome.
The frequency, dilution of stool, the appearance or increase in the amount of pathological impurities compared to the usual frequency and character of stool in a given child during antibiotic therapy (in the absence of other reasons) was regarded as the development of antibiotic-associated diarrhea.
Criteria for evaluation
Symptoms typical of AAD, such as profuse loose stools, intestinal colic, flatulence, and vomiting, were assessed as effectiveness criteria. The severity of symptoms of the disease was assessed in points: 0 - absent, 1 - moderately expressed, 2 - significantly expressed.
Dosage
The drug was dosed according to the instructions: 1/2 capsule 2 times a day 40 minutes before feeding. The contents of the capsule were mixed with breast milk or formula. The drug Lactovit forte was prescribed from the first days of antibiotic therapy.
All children included in the study were examined using clinical and laboratory methods. Maternal complaints and objective data were assessed, including body temperature, frequency and consistency of stool, the presence of pathological impurities in stool, abdominal volume, body weight dynamics; laboratory tests (general blood and urine analysis, coprogram, bacteriological examination of stool).
Portability
The tolerability of the drug was assessed based on subjective and objective data obtained during treatment. Tolerability was assessed in points: 1 - extremely unsatisfactory, 2 - unsatisfactory, 3 - satisfactory, 4 - good, 5 - very good.
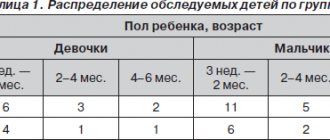
The study included 60 newborn children, including 34 boys and 26 girls. The average age at the time of initial examination was 8.4 days (range 3 to 16 days of life).
All children were randomly divided into 2 groups. Group I (main) consisted of 30 children who received the study drug Lactovit Forte along with antibiotic therapy, group II (control) included 30 children who received basic therapy without a probiotic drug.
The distribution of children by underlying disease is presented in Table. 1.
Results and discussion
All patients, depending on the underlying disease, received a course of antibiotic therapy lasting from 7 to 25 days. The majority of children received cephalosporins - 13 in the main group (43.3%) and 15 children (50%) in the control group; 10 (33%) from the main group and 9 children (30%) from the control group received a combination of cephalosporins with aminoglycosides. Semi-synthetic protected penicillins were received by 7 (23.3%) and 6 (20%) children in the main and control groups, respectively.
An assessment of risk factors for the development of antibiotic-associated diarrhea was carried out. The main factors considered were prematurity, the type of antibacterial agent used, tube feeding, and whether the child was on a ventilator. An analysis of the influence of risk factors on the incidence of AAD is presented in Table. 2.
In the control group, AAD developed in 19 children (63%), while in the main group - only in 5 children (16.7%). The significant significance of reducing episodes of diarrhea when taking the probiotic Lactovit forte was confirmed - RR = 0.26 (95% confidence interval (CI) 0.11–0.61).
Antibiotic-associated diarrhea occurred on average at 4.4 ± 1.7 days in newborns (95% CI 3.7–5.1). There were no significant differences in the time of occurrence of AAD in the study and control groups (p > 0.05).
The most significant factors in the development of AAD were prematurity, tube feeding, mechanical ventilation and the use of a combination of cephalosporins with aminoglycosides.
The duration of AAD symptoms in the control group was 4.7 ± 1.2 days (95% CI 4.2–5.3), while in the main group the average duration was 2 times shorter and amounted to 2.2 ± 0.8 days (95% CI 1.2–3.2). According to the results of the analysis, a significant difference in the duration of AAD in the main and control groups was found, which reflects the therapeutic effectiveness of the probiotic Laktovit forte in relation to the duration of the disease.
In Fig. Figure 1 shows the dynamics of the main clinical symptoms of AAD in newborns, expressed in points. In children of the main group, on the 3rd day of assessing clinical manifestations, the average score of typical symptoms of AAD was significantly (P < 0.01) lower in relation to newborns of the control group - 4.2 ± 1.0 points versus 6.0 ± 1.2 points; on the 7th day - 1.8 ± 0.8 points versus 3.1 ± 1.3 points. By the 10th day, no clinical manifestations were noted in the newborns of the main group (Fig. 1).
A good assessment of tolerability (4 points) of the drug Lactovit forte was observed in 2 children, the remaining 28 children had very good tolerability of the drug (5 points). Throughout the study, no side effects were observed when using the probiotic Lactovit Forte in newborns.
conclusions
1. The incidence of AAD in newborns is 63%, which exceeds the incidence among other age groups; this determines the need for preventive measures using probiotic preparations.
2. Risk factors for the development of AAD in newborns were: prematurity, mechanical ventilation, tube feeding, and the use of a combination of antibiotics.
3. The use of the probiotic Lactovit forte, containing B. coagulans , reduces the risk of developing AAD in newborns by almost 3.5 times, reduces the duration of diarrhea syndrome and reduces the degree of clinical manifestations of intestinal dyspepsia in children during the use of antibiotics.
Duration of use:
The course of treatment depends on the nature and course of the disease. If there are intestinal disorders, treatment is carried out for at least 4-6 weeks; for chronic colitis of various etiologies, treatment is carried out for up to 1.5-2 months; for dysbiosis resulting from the use of antibiotics, sulfa drugs and other reasons, treatment is carried out for 3-4 weeks.
To consolidate the obtained clinical effect, 10-14 days after the end of the course of treatment, in the absence of complete normalization of the microflora, maintenance doses of the drug are prescribed (half the daily dose) for 1-1.5 months.
For diseases that occur with relapses, repeated courses of treatment are advisable. In these cases, before prescribing the drug, a repeated study of the microflora is necessary.
Side effect.
From the digestive tract: dyspepsia, nausea, vomiting, flatulence, abdominal discomfort, loss of appetite, diarrhea. From the skin and subcutaneous tissue: redness of the skin, itching, skin rashes, rash, urticaria, acne, bullous rash. From the immune system: hypersensitivity reactions (including anaphylaxis, anaphylactic shock, angioedema, bronchospasm). From the nervous system: headache, dizziness, nervous agitation. From the heart: pain in the heart area, tachycardia, peripheral vascular thrombosis. Others: hot flashes, general weakness, sweating, hyperthermia.
Interaction with other drugs.
Folic acid. The absorption of folic acid decreases when used simultaneously with analgesics, anticonvulsants, antacids, chloramphenicol, neomycin, polymyxins, antibiotics, sulfonamides, and cytostatics.
Folic acid increases the metabolism of phenytoin. In patients with folate deficiency, the use of folic acid may reduce the plasma levels of phenobarbital, phenytoin and primidone and cause an epileptic seizure. Oral contraceptives, ethanol, sulfasalazine, cycloserine, glutethimide, and methotrexate may affect folate metabolism.
Do not use together with mineral acids, alkaline substances, reducing agents, since folic acid is inactivated.
Decreased or altered absorption may occur with concomitant use of cholestyramine and folic acid. Therefore, the drug must be taken 1 hour before or 4-6 hours after taking cholestyramine.
Cyanocobalamin. Aminoglycosides, salicylates, antiepileptic drugs, colchicine, potassium preparations reduce the absorption of cyanocobalamin and affect its kinetics. When used simultaneously with kanamycin, neomycin, polymyxins, tetracyclines, the absorption of cyanocobalamin is reduced.
When used simultaneously with thiamine, the risk of developing allergic reactions caused by thiamine increases.
Chloramphenicol reduces the hematopoietic response to the drug.
When used simultaneously with citamen, the effect of citamen is reduced. Oral contraceptives reduce the concentration of cyanocobalamin in the blood.