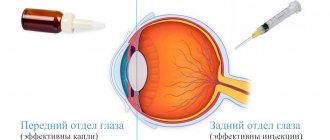
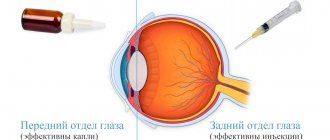
Conservative treatment methods are an integral component of the treatment program for eye diseases. One type of such treatment is intravitreal injections - a method in which medications are injected directly into the eye cavity through injections. This type of treatment is indicated for patients with pathologies of the posterior part of the eye (retina, optic nerve, vitreous) due to the fact that drops and tablets for these diseases are much less effective.
A drug introduced into the eye cavity requires the use of a smaller amount and a lower concentration of the drug substance, while it becomes possible to preserve the medicinal properties of the drug for a long time (from two weeks to two months) directly at the site of the lesion, in this case - inside the eye.
Our specialized ophthalmology center is one of the most experienced medical institutions providing services to patients with retinal pathologies. Every month, hundreds of microsurgical operations are performed within the walls of our clinic; they are performed by highly qualified specialists with many years of clinical experience.
Pharmacodynamics and pharmacokinetics
It has been established that Vitreous injections are prescribed when resorption of postoperative scars or burn scars is required, as well as to reduce pain caused by various neuralgic diseases, for example, radiculitis . This substance, obtained from the eyeball of an animal, contains various amino acids necessary for the formation of muscle tissue. In addition, the substance contains hyaluronic acid , which ensures the normal functioning of heart valves and joints.
Despite the fact that this drug is a natural product of animal origin, there are a number of contraindications for its use, so treatment is carried out only after consultation with a doctor.
How does the procedure work?
In terms of their effectiveness, inravitreal injections are comparable to some types of vitreoretinal operations. The procedure requires sterile conditions and therefore must be performed in a specially equipped suitable room, such as an operating room. To perform injections into the eyeball, local anesthesia is used with eye drops, in order to avoid complications, the patient is prescribed antiseptics.
During the administration of the drug into the eye cavity, an anesthesiologist is present in the operating room, who monitors the patient’s vital functions and ensures the proper level of pain relief for the patient. The requirements for asepsis and antisepsis during intravitreal injection manipulation are the same as when performing vitreoretinal surgery and must comply with international standards for intraocular surgical interventions.
At the initial stage, the patient's eyeball is fixed using an eyelid expander, after which the doctor marks the area for future intraocular injection in the lower outer quadrant of the eyeball, 3.5 mm from the limbus. This allows the doctor to avoid future trauma to intraocular structures (lens, ciliary body, retina) and reduce the likelihood of intraocular complications. The drug is injected directly into the vitreous body of the eye through the outer membranes of the eye; for this purpose, special needles are used to prevent the drug from leaking out when the needle is removed. Due to the fact that the manipulation is carried out using an operating microscope, the doctor controls the process of drug administration and the depth of needle insertion. All of the above points make the procedure safe and minimize the risks of complications. Equally important is strict adherence to the recommendations of the attending physician in the postoperative period and regular postoperative monitoring.
Our clinic is equipped with the most modern equipment; we provide European-level services without queues and at affordable prices. When performing medical procedures, our specialists use the best equipment available today, including disposable instruments.
Indications for use
The drug Vitreous is prescribed:
- for the purpose of softening or resolving scar tissue formed as a result of burns , surgical interventions, and so on;
- for rapid formation of callus during fractures ;
- when you need to reduce pain due to radiculitis, neuralgia ;
- and also to improve joint mobility, for example, with contractures and other cases.
Treatment
Initial and slowly progressing variants of DST, as a rule, do not require treatment - especially since effective drug regimens have not yet been found (in such cases, as a rule, ophthalmologists prescribe “vascular eye drops” - Emoxipin or Taufon, vitamins for vision, enzyme drugs in tablets - Wobenzym).
In the worst case, and under the condition of constant conscious (or unconscious, neurotic) fixation of attention on them, floating threads or spots can become a source of psychological discomfort, without objectively affecting either visual acuity or other characteristics of the visual system. However, in most cases, the natural development of the process (for example, a gradual increase in age-related changes) leads to the gravitational lowering of inhomogeneities under the main optical axis of the eye, i.e. “floaters” simply stop creating interference in the most active central field of view. At the same time, the visual centers of the cerebral cortex gradually adapt to the existence of persistent interference and begin to ignore them, filtering them out and excluding them from the final visual image.
If the destruction of the vitreous body is a consequence of a more general and serious pathology, for example, inflammation of the retina, diabetic retinopathy, etc., such trends certainly require diagnosis, constant monitoring and treatment (at least preventive or supportive) by an ophthalmologist . In the most advanced, rapidly progressing and threatening variants of the development of DST, vitrectomy is performed - an ophthalmic surgical operation to partially or completely remove the vitreous substance and replace it with a balanced biocompatible salt solution, the physical and optical parameters of which correspond to the natural characteristics of a healthy vitreous.
Not being absolutely safe in terms of risks and possible complications (however, like any other surgical intervention practiced today), vitrectomy is considered as an ultima ratio, i.e. as a “last argument”, a forced measure to preserve vision, and ends in therapeutic success in a very large percentage of cases - according to various estimates, from 80% to 90%.
Contraindications for use
The use of the drug is not recommended for:
- infectious diseases;
- acute inflammatory processes;
- cachexia;
- jade;
- nephrosclerosis;
- liver cirrhosis;
- congestive heart failure;
- malignant tumors.
Symptoms of the disease
The presence of this pathology can be recognized by the following signs and symptoms:
- Along with the appearance of interference, the quality of vision sharply deteriorates .
- The appearance of floating clouds before the eyes and their number increasing over time.
- Loss of the field of vision (a veil appears before the gaze).
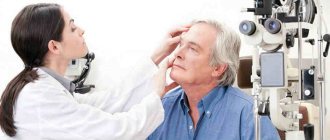
Important! If such signs appear, it is necessary to urgently contact an ophthalmologist, who, by examining the vitreous body, will classify the disease and prescribe appropriate treatment.
Reviews about Vitreous humor
In most cases, reviews about the vitreous body are found on forums related to the treatment of gynecological diseases. However, many patients simply do not understand why they were prescribed this drug. Therefore, they try to consult with specialists online or ask other women who were once prescribed similar treatment.
In addition, some users are confused by the fact that they were prescribed Vitreous intramuscularly, since the instructions indicate that it is used subcutaneously. However, experts confirm that the drug can be used intramuscularly, but if such a prescription seems doubtful to patients, then it is necessary to clarify its correctness with your doctor or another doctor. Also, some women are concerned about how this drug affects the development of the fetus if pregnancy begins during treatment.
According to experts, this particular drug does not have a negative effect on either the woman’s body or the development of the fetus. But since it is usually prescribed as part of complex therapy, the effects of other drugs must also be considered. In any case, all these questions must be clarified at a doctor’s appointment, during a face-to-face consultation.
What drugs are used for intravitreal administration?
The most commonly used drugs for administration into the eye cavity are Lucentis, Ozurdex, Kenalog, Aylia and Gemaza. Antibacterial drugs can also be used for intravitreal administration.
The drug Lucentis is used to treat the wet form of age-related macular degeneration (AMD), as well as in patients with neovascular membrane (due to previous inflammation of the eye, high myopia, etc.), diabetic retinopathy, and occlusions of the central retinal vein. The action of the drug Lucentis is aimed at preventing the growth of abnormal defective vessels and associated complications in the form of multiple hemorrhages in the eye cavity, retinal detachment and loss of vision. In most cases, although not 100% of cases, Lucentis helps stop the progression of visual acuity deterioration and prevent blindness. For the same purpose, intravitreal administration of the drug Aylia is used.
Ozurdex is a drug used to treat macular edema. The dosage form of the drug Ozurdex - an implant developed for intravitreal administration - ensures long-term preservation of the therapeutic effect. Thanks to this, a prolonged, powerful anti-inflammatory and anti-edematous effect is achieved, which contributes to long-term improvement of vision.
Kenalog is a glucocorticosteroid for intravitreal administration that has an inhibitory effect on all phases of the inflammatory process in the eye cavity. In ophthalmology, the drug is used for administration parabulbarly and into the sub-Tenon's space. Kenalog is characterized by a prolonged therapeutic effect and the absence of a systemic effect on the body.
Gemaza in the form of intravitreal injections is used to treat occlusion of the main vessels of the retina, hemorrhage in the eyeball (hemophthalmos, hyphema) and in the retinal area, adhesions after surgical treatment of glaucoma. The drug provides fibrinolytic and thrombolytic effects.
Vitreous body price, where to buy
The price of Vitreous in pharmacies averages 1250-1300 rubles.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
LuxPharma* special offer
- Vitreous body in ampoules No. 10
RUR 2,400 order
show more
Pharmacy24
- Vitreous body 2 ml N10 solution TOV FZ BIOPHARMA, Ukraine / PrAT Biopharma, Ukraine
102 UAH order
PaniPharmacy
- Vitreous ampoule Vitreous solution d/in. amp. 2ml No. 10 Ukraine, Biopharma CJSC
112 UAH order
show more
Vitreous body. Structure, pathology and methods of surgical treatment (literature review)
The review describes the anatomical characteristics of the vitreous body, general symptoms of its pathological changes, as well as modern possibilities for surgical treatment of diseases of the vitreous body. Key words: vitreous, pathology of the vitreous, surgical treatment of the vitreous. Abstract Vitreous. Anatomy, pathology and methods of surgical treatment (literary review) Alekseev IB, Belkin VE, Samoylenko AI, Gularia AA
Clinical Hospital named after SP Burdenko Branch No. 1 Anatomic characteristics of vitreous, symptoms of its pathology and modern surgical methods of its treatment are described in the article. Key words: vitreous, vitreal pathology, vitreous surgery.
In the pathology of the eyeball, along with lesions of the membranes, lens and optic nerve, changes in the vitreous body are of no small importance. Violations of transparency, tone, and sometimes its volume often lead to decreased function, blindness, and even atrophy of the eyeball. In many cases, the outcome of injuries, inflammatory and dystrophic processes, as well as the result of surgical intervention, depends on the condition of the vitreous body. However, in the practice of ophthalmologists, the vitreous body receives much less attention than any other part of the eye. In textbooks and manuals, as a rule, only a few pages are devoted to the description of the vitreous body and its pathology. This situation was not accidental until recently. It was due to the paucity of manifestations of vitreous pathology detected by conventional clinical research methods, the “secondary” nature of the lesions, and the lack of sufficiently effective methods for treating the detected changes.
The vitreous body is a transparent, colorless, gelatinous mass that fills the cavity of the eyeball, limited in front by the lens, zonular ligament and ciliary processes, and throughout the rest of the length by the retina. The vitreous body is the most extensive formation of the eye, making up 55% of its internal contents. Its volume in an adult is 3.5-4 ml, weight - about 4 g.
Anatomically, the vitreous body has three components: the vitreous body itself, the limiting membrane and the cloquet canal. In the vitreous itself, some authors distinguish the cortical and nuclear sections (Ivanov, 1865), others distinguish three sections: adjacent to the lens - pars retrolentalis, to the ciliary body - pars ciliaris and to the posterior pole of the eye - pars posterior (Schreck, 1958). G.L. Starkov points out that in clinical interests it is advisable to conditionally differentiate the vitreous body, distinguishing the anterior section, which unites the retrolental and ciliary zone and spatially constitutes the anterior third of the vitreous mass, the central section, representing its middle third, and the posterior section adjacent to the posterior segment of the fundus . This division, in particular, is convenient for localizing vitreal changes detected during biomicroscopy (1964) [3].
In some areas, the vitreous body is quite tightly connected with the tissues of the eye that limit it. It is most firmly fixed in front of the dentate line at the flat part of the ciliary body and the posterior fibers of the zonular ligament. Sallmann called this belt-shaped area 2-2.5 mm wide the base of the vitreous body - basis corporis vitrei (1953) [13]. Some authors (Schepens, Busacca), based on the frequent detection of adhesions over a wider space, are inclined to believe that the border of the vitreal base extends to the ora serrata (1956) [4, 5, 16]. Rao understands the basis of the vitreous body as all its fusions with the retina, especially in the area of vitreoretinal vascular anastomoses (1955) [14]. Teng, Chi and Gartner found a circular band-like connection of the vitreous body with the retina 3 mm posterior to the ora serrata and also attributed it to the vitreal base. According to the authors' description, the fixation line has the appearance of a delicate grayish-white stripe adjacent to the surface of the retina and characterized by arcado-shaped contours. The authors warn against mistaking this line for dystrophic changes in the retina (1962) [6, 18].
The posterior zonular fibers entering the vitreous body from the front strengthen its attachment to the ciliary body and lens, forming a kind of circular ligament - lig. hyaloideum capsulare. The attachment point of this ligament to the lens is referred to as Vieger's ring. Goldman unites lig. capsulare and Viger's ring into one common formation, calling it lig. Viger's hyaloideum capsulare, and believes that it is a circular attachment to the lens of the anterior limiting membrane of the vitreous body (1962) [7, 8]. Inside Wieger's ring between the lens and the vitreous body there is a capillary gap - the retrolenticular space (1957) [4, 10, 11]. The second most durable place of attachment of the vitreous body to the wall of the eye is located at the optic nerve head along the border of the area Martegiani. This name refers to the funnel-shaped depression in the vitreous body described by Martegiani in 1814, located in front of the optic disc and, as it turned out later, representing the beginning of the cloquet canal. It looks like a ring, the diameter of which is equal to the diameter of the optic nerve head.
Regarding the fixation of the vitreous body to the retina along the remaining length between the ora serrata and the optic nerve head, the opinions of researchers are contradictory.
Many people believe that here the vitreous body is only closely adjacent to the retina. In contrast, Redslob finds that it is everywhere connected to the retina by thin fibers (1932) [15]. Nordenson and Khrushchev also describe the attachment of the vitreous body to the inner surface of the retina along its entire length (1926) [12]. Schreck speaks of adhesions in the macula, equator, and other places near the vessels; equatorial fusions are reported by Grignolo (1958) [9]. Hagedorn and Sieger (1956), Teng and Chi (1957) draw attention to frequent histological findings in healthy eyes of a significant number of small limited adhesions of the vitreous body to the retina in various places. Calling these adhesions “rosettes,” the authors consider them congenital and attach a certain significance to them in the pathogenesis of retinal detachments. Having a gel-like consistency, the vitreous exactly repeats the shape of the eye cavity it fills. On the anterior surface of the vitreous body there is a depression - a cup-shaped fossa (fossa patellaris), formed by depression of the posterior part of the lens. The vitreous body taken out of the eye almost does not blur, which indicates the presence of a skeleton in it.
The consistency of the vitreous in different parts of the human eye is not the same: in freshly enucleated eyes it appears more liquid in the middle than at the periphery. When the vitreous is cut into the newly enucleated eye, a small amount of fluid is released. However, postmortem changes begin very early, as a result of which all or almost all of the vitreous quickly becomes liquid. This post-mortem liquefaction poses a serious difficulty for the study of vitreal structure (1964) [3].
On macroscopic examination, the vitreous body appears homogeneous. The absolute transparency of the vitreous body has led to contradictory information about its structure, and even the presence of any structure in the vitreous body is questioned.
Nevertheless, some fairly simple techniques, such as dissection of the frozen vitreous, make it possible to macroscopically determine its architectonics, identify the membrane, as well as the cloquet canal enclosed in it. This technique formed the basis for all studies of macroscopic vitreal structure.
The key point in studying the structure of the vitreous body was the works of Worst and Z.A. Makhacheva. By injecting dyes into isolated human vitreous humor, Worst (1977) discovered and first described sac-like cavities and called them “cisternae” [19]. Depending on the location, he identified circulus cisternalis retrociliaris (ring of retrociliary cisterns), circulus cisternalis equatorialis (ring of equatorial cisterns), circulus cisternalis petaliformis (ring of petaliform or (in English) petal cisterns).
Bursa premacularis (premacular bursa) was first described by Worst in 1975 as a pear-shaped sac-like cavity located in close proximity to the macula of the retina (2006) [2].
Cisterna preoptica (preoptic tank) is a small cavity located in close proximity to the optic disc and corresponding to the spatium prepapillare Martegiani (prepapillary space Martegiani, 1814) (2006) [2].
Retrociliary cisterns are cylindrical cavities that communicate with each other and form a ring in the projection of the ciliary body. When dye is introduced into them, the tanks are gradually filled in the form of a circle with joining ends. Along the periphery, the ring of retrociliary cisterns is connected to the dense substance that makes up the formative framework of the vitreous body. The retrociliary cisterns are located in the form of a ring on the anterior, somewhat concave surface of this dense frame; the equatorial and petaliform cisterns are located in its thickness, oriented around the central cone of the vitreous body formed by the canals (2006) [2].
By dissecting the vitreous body, it is possible to reach the posterior pole, which is represented by the most important formations both anatomically and functionally: the premacular bursa and the preoptic cistern. On the outside, these formations are surrounded by a dense ring of cortical casing, covered on the outside with a hyaloid membrane (2006) [2].
The premacular bursa is a closed cup-shaped cavity. In this zone, the vitreous cortex is extremely thin and is practically absent in the area corresponding to the central fovea. This explains the extreme vulnerability of the prefoveal parts of the vitreous body, when damaged, its internal structures fall out (2006) [2].
BEHIND. Makhacheva established that the anterior wall of the premacular bursa is formed by an intravitreal membrane with numerous holes that give it the appearance of a “sieve” - the cribriform membrane (membrana cribrosa Makhacheva). The posterior wall is formed by a thin limiting membrane, which is internally covered with a spongy layer (stratum spongiosum Makhacheva), with the exception of the area corresponding to the foveal zone of the retina. The spongy substance is easily removed from the surface of the posterior wall of the premacular bursa using the transvitreal approach (2006) [2].
The posterior wall of the premacular bursa is connected to the underlying internal border plate of the retina through a circular ligament called Worst (by analogy with the hyaloid-capsular ligament) hyaloid-macular. Also, by analogy with the retrolental space, between the posterior wall of the premacular bursa and the internal border plate of the retina there is a slit-like subbursal space filled with clear fluid (2006) [2].
The premacular bursa and prepapillary space are connected to the anterior parts of the vitreous body through canals (2006) [2].
Based on his research Z.A. Makhacheva came to the conclusion that the central, so-called cloquet canal, connects the retrolental space directly with the premacular bursa, and not with the prepapillary region, as is commonly believed (Duke-Elder, 1961) (2006) [2]. The dye, located in the central canal and the premacular bursa, penetrates into the prepapillary space through the connecting canaliculus (canaliculus communicans Makhacheva), which connects two systems of vitreous canals (2006) [2].
Along with the central canal, which is more correctly called the lenticomacular canal, there is a canal in the vitreous body that connects the prepapillary space with the retrociliary cisterns of the presumably superior nasal segment. This channel is named Z.A. Makhacheva optico-ciliary (canalis optico-ciliaris Makhacheva) (2006) [2].
The lenticomacular canal communicates with the cisterns surrounding it on all sides, and is similar to a tree trunk with a dense crown. Anastomoses of the optociliary canal are not so extensive and are limited to a small zone around its mouth in the area of the retrociliary cisterns (2006) [2].
The channels, in all likelihood, perform an exchange-transport function, regulating the directional movement of fluid flows in the vitreous body and maintaining the metabolic and hydrodynamic balance between the anterior and posterior parts of the eye (2006) [2].
The existence of a canal connecting the retrociliary cisterns with the prepapillary space makes it possible to rethink the mechanism of glaucoma (2006) [2].
The direct connection between the retrolental region and the premacular bursa explains the occurrence of complicated cataracts with involutional and inflammatory lesions of the macula, as well as complications in the posterior segment of the eye after removal of the lens and antiglaucomatous operations (2006) [2].
On the inner walls of the central canal and on the anterior surface of the retrociliary cisterns Z.A. Makhacheva was able to identify thin tubule-like structures that may be involved in the circulation of vitreal fluids. For a final conclusion about these anatomical formations, further studies using scanning and transmission electron microscopy (2006) are necessary [2].
The channels are interconnected by means of a connecting canaliculus; they could be clearly traced during retrograde cannulation of the prepapillary space and premacular bursa (2006) [2].
It is noted that when the dye is introduced into the prepapillary space, the contrast agent fills the optociliary canal, and then spreads through the connecting tubule into the premacular bursa and the lenticomacular canal. During retrograde cannulation of the premacular bursa, the dye first fills the lenticomacular canal, then through the connecting canaliculus penetrates into the prepapillary space and much later, with additional injection of dye, into the optociliary canal (2006) [2].
There may be some valve or mechanism that impedes the retrograde filling of the optociliary canal from the premacular bursa, which ensures unidirectional fluid movement, and disruption of this mechanism may contribute to the occurrence of glaucoma (2006) [2].
The presence of a constant flow of fluid in the vitreous body was also confirmed by the results of radiographic studies: the movement of indifferent dyes or radionuclide isotopes introduced extraocularly in the vitreal masses was established. The fluid produced by the ciliary body enters the base of the vitreous body, from where it moves along the outflow paths anteriorly - into the anterior chamber and posteriorly - into the perivascular spaces of the optic nerve. In the first case, the liquid mixes with the chamber humor and is removed along with it; in the second, from the posterior part of the vitreous body, bordering the optical part of the retina, the liquid flows through the perivascular spaces of the retinal vessels. Knowledge of the circulation of intraocular fluid allows one to imagine the nature of the distribution of medicinal substances in the eye cavity (2002) [1].
The study of the vitreous body in normal and pathological conditions with the help of modern slit lamps, which make it possible to observe it to its full depth, significantly expands previous ideas about it. Often the vitreous body, transparent when observed with an ophthalmoscope, biomicroscopically appears to be significantly altered. The nature of these changes, as well as more severe lesions visible even with ophthalmoscopy, is very diverse. Each form of pathological condition of the vitreous body has quite clearly defined clinical and biomicromorphological signs, is represented by more or less typical dynamics, and is sometimes associated with certain general diseases of the eye.
In the current state of the doctrine of vitreal pathology, the term “vitreous opacification” can no longer satisfy. To limit ourselves in defining changes in the vitreous body only to this term is now tantamount to reducing the richest pathology of the cornea, iris or lens to the designations “keratitis”, “iritis”, “cataract” without reflecting the morphological, etiological and other features of each case in the diagnosis, i.e. without indicating the form of the lesion.
Due to the unique structure, physiology and functions of the vitreous body, its changes, as a rule, are found in eyes with more or less clear signs of an inflammatory or dystrophic process. It is still far from always possible to answer the question whether vitreal lesions are secondary in such processes or arise in parallel with pathological changes in the membranes from the action of one common cause. At the same time, it is not uncommon for cases when, with severe pathology of the vitreous body, it is not possible to detect any signs of suffering in the surrounding parts of the eye. There are many clinical forms of vitreal changes, but general symptoms of vitreous lesions can be identified. These include its liquefaction and disturbances in transparency, since these phenomena are observed in almost all forms of vitreal pathology.
Liquefaction of the vitreous
At the onset of the disease, liquefaction, as a rule, occurs in the central section, mainly in the layers adjacent to the tractus hyaloideus. Cavities occupied by the liquid part of the vitreous can form here. Gradually expanding and merging, they capture more and more space, spreading to the peripheral sections.
Clinically, the main diagnostic sign of liquefaction of the vitreous body is the increased mobility of the opacities present in it or the fibers of its skeleton. Sufficient intensity of opacities makes it possible to establish liquefaction even when examining in transmitted light or ophthalmoscopically.
Be that as it may, liquefaction of the vitreous body always indicates pathology and necessitates finding out its causes. In some cases, this can facilitate timely diagnosis of developing eye disease. Determining the consistency of the vitreous is practically important when performing many intraocular operations, since timely measures taken can prevent or reduce the loss of liquefied vitreous. The process of liquefaction of the vitreous body is irreversible [3].
Vitreous opacification
Involvement of the vitreous body in the pathological process is almost always accompanied by more or less pronounced disturbances in its transparency. The opacities that arise in this case have features determined both by the properties of the vitreous body and the nature of those eye diseases as a result of which it changes. The shape, size, quantity, intensity, mobility and location of opacities are very diverse, as are the causes that cause them. Opacities can be congenital or acquired. The latter, in turn, are exogenous or endogenous in nature. At the present stage of development of ophthalmology and microsurgery, it becomes clear how important the role of the vitreous body is in the formation of pathological changes in the eyeball, often leading to blindness and loss of the eye as an organ. Today, the only high-quality treatment for complicated vitreous pathology is vitrectomy. Vitreous removal requires a thorough understanding of the anatomy of the ocular structures and must be based on a systematic approach. The vitreous should be considered in terms of discrete surfaces that are removed in a specific order. The goal of vitrectomy should not be to remove part of the vitreous to visualize the posterior pole or only nuclear vitrectomy, but the intervention should be aimed at eliminating the underlying vitreal pathology process. If vitrectomy is performed using a vitreotome with a cutting knife and controlled aspiration, the surgeon is able to remove most of the vitreous without moving the vitreotome from the center of the vitreous cavity. This is possible due to the high speed of aspiration and because the knife pulls the vitreous towards the vitreotome; however, such tractions are now recognized as dangerous. Because of this traction-inducing movement of the vitreous toward the center, the erroneous concept of “central vitrectomy” has arisen. In fact, many patients with significant vitreoretinal pathology who require vitrectomy do not even have a “central” vitreous. In cases of recent trauma, in rare cases of recent retinal detachments, and in the presence of macular holes, the vitreous may be relatively normal, but the patient requires central vitrectomy.
Sharper vitreotomes, the high speed of their cuts, regulators that provide rapid aspiration, and its proportional control contribute to the removal of the vitreous without moving it from its original position.
When performing vitrectomy, in our experience, the anterior base of the vitreous body should be removed first and without fail, starting from the center to the periphery. All attachments to the posterior lens capsule, anterior segment tears, or iris must be removed before proceeding posteriorly.
Inserting an infusion cannula through the pars plana allows the surgeon to interchange the vitrectomy instruments and endoilluminator, allowing access to the entire posterior curvature of the lens. Removal of the anterior base of the vitreous in phakic eyes requires direct microscopic visualization and additional coaxial endo-illumination without the use of corneal contact lenses or a wide-angle system to avoid damage to the lens.
After removing the anterior base of the vitreous and its central part, the second task is usually to remove the posterior base of the vitreous. Before performing the intervention, it is necessary to obtain information about the presence of posterior vitreous detachment using ophthalmoscopy or ultrasound diagnostics. If there is a need to perform vitrectomy through the pars plana in an eye where, as a rule, there is complete vitreoretinal contact, a partial posterior vitreous detachment with its conical configuration, or a complete posterior vitreous detachment with a frontal flat configuration, the entrance to the posterior base of the vitreous should be be performed from the nasal side.
All parts of the posterior base of the vitreous not in contact with the retina, both moorings and conical surfaces, must be removed to free the retina from traction. However, the remnants of the vitreous that are the leading edge of the truncated cone, the so-called “skirt,” must be removed differently. Traction from the skirt can lead to retinal rupture and must be sufficiently removed to ensure optimal fundus visualization during surgery and to prevent the skirt from obstructing any part of the visual field when the body is upright.
In conclusion, I would like to note that the vitreous body undeservedly receives so little attention in the literature. Its structure and the processes occurring in it play a huge role in the normal functioning of the eyeball. That is why it is important to understand the pathological changes occurring in it.
Literature 1. Kopaeva V.G. Eye diseases: Textbook. M.: Medicine, 2002. 560 p. 2. Makhacheva Z.A. Anatomy of the vitreous body: a textbook for the system of postgraduate professional education of doctors. M.: Rusprint, 2006. 16 p. 3. Starkov G.L. Pathology of the vitreous body during biomicroscopic examination. Diss. Novokuznetsk, 1964. 4. Busacca A. Biomicroscopie und Histopathologie des Auges, 1956. 5. Busacca A., Goldmann H. Schiff-Wertheimer S. Biomicroscopie du corps vitre et Du Fond de l'oeil. Paris, 1957. 6. Gartner Kl. Mbl. //Augenheilk. 1962. Vol. 140. R. 524–544. 7. Goldmann H. // Ophthalmologica. 1954. Vol. 127. R. 335. 8. Goldmann H. // Ophthalmologica. 1962. R. 143. 9. Grignolo // Arch. Ophthal. 1952. Vol. 47. R. 760. 10. Koeppe L. // Arch. Ophthal. 1918. Vol. 95, 3; 96, pp. 199–231. 11. Koeppe L. Die mikroskopie der lebenden hinteren Augenhalfte in Naturlichenlichte. Berlin, 1922. 12. Nordenson. Die Netzhautablosung. Wiesbaden, 1887. 13. Rossi A. // Brit. J. Ophthal. 1953. Vol. 37/6. R. 343. 14. Rao S., Kulkarni M., Cooper S. u. Radhakzishnen // Brit. J. Ophthal. 1955. Vol. 39. R. 163–169. 15. Redslob E. La corps Vitre. Paris, 1932. 16. Schepens C. // Am. J. Ophthal. 1955. Vol. 39. R. 631–633. 17. Schimek, Steffensen P. // Am. J. Ophthal. 1955. Vol. 39. R. 677–683. 18. Teng C., Chi H. // Am. J. Ophthal. 1957. Vol. 44/3. R. 335–386. 19. Worst JGF, Los LI Cisternal Anatomy of the Vitreous. Kugler Pub. Amsterdam, 1995.