What is known about Ultrix Quadri?
This is an inactivated split vaccine containing antigens of influenza virus types A (H1N1 and H3N2) and B (Yamagata and Victoria lines). Thus, unlike the regular Ultrix, it is four-part. Vaccines of this type appeared in Russia 2 years ago, in 2022. They are considered more effective (as they protect against more varieties of influenza), but production capacity was initially sufficient only for risk groups (people with severe chronic diseases), so they became available to a wide range of patients relatively recently. It is now rated as one of the most reliable flu shots available to Russians. The vaccine was created by the Rostec state corporation.
Vaccine prevention of influenza. New domestic vaccine Ultrix®
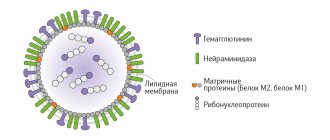
Influenza A viruses are the most contagious and virulent; they contain 2 types of neuraminidase (Na1, NA2) and 3 types of hemagglutinin (HA1, HA2, HA3). The influenza B virus contains 1 type of neuraminidase and 1 type of hemagglutinin. Virus C does not contain neuraminidase, but contains hemagglutinin, has a receptor-degrading enzyme - neuraminate-O-acetylesterase and does not have variability. A change in NA or HA is called an antigenic shift. Intermediate hosts and natural reservoirs of influenza A virus can be birds (bird flu). Virus variability may be associated with point mutations in the genome and changes in NA or HA (antigenic drift). It is possible to completely replace HA and NA through the mechanism of reassortment/recombination (antigenic shift), which occurs once every 10–12 years and can lead to the development of a pandemic. Influenza pandemics include: Spanish flu (H1N1) in 1918, Asian flu (H2N2) in 1957, Hong Kong flu (H3N2) in 1968. With antigenic drift, about 10% of the population gets sick, with antigenic shift – 40– 60%, because there is no immunity to the new type of virus [1, 2]. According to WHO, an adult can get respiratory infections 3–4 times a year, children attending school – 4–5 times, preschoolers – up to 6 times, children in the first year of life can get sick 2–12 times a year [3]. The last influenza epidemic was observed in 2009, it was caused by the influenza A (H1N1) pdm 09 virus. Around the world, 220 thousand people fell ill, of which 1900 people died [3, 4]. During the epidemic season of 2012–2013. influenza A (H1N1) pdm 09 virus was already circulating as a seasonal virus, along with other influenza A (H3N2) and B viruses [3, 16]. In Russia during the 2013–2014 epidemic season. 138 deaths from laboratory-confirmed influenza were registered. Influenza A (H1N1) pdm 09 virus was isolated from 135 deceased people, influenza A (H3N2) virus was isolated from 1 person, and influenza B virus was isolated from 1 person. Endocrine pathology was diagnosed in 40.8% of the deceased, obesity in 24.8% and obesity in 37 .6% – diseases of the cardiovascular system [3].
Specific influenza vaccine prevention is quite effective. Table 2 lists the most commonly used vaccines. The vaccines have similar immunogenicity and their composition changes every year, according to WHO forecast. The domestic influenza vaccine is a trivalent polymer-subunit vaccine, it contains polyoxidonium, a high-molecular adjuvant associated with influenza virus A (H1N1, H3N2) and B antigens - neuraminidase and hemagglutinin. Polyoxidonium enhances the formation of protective immunity, despite the reduced content of influenza virus antigens in the vaccine. Ultraviolet light is used to inactivate the virus, which increases the safety of the vaccine. Influenza is approved for use from 6 months, is included in the national vaccination calendar, is a low-reactogenic and highly purified vaccine, its safety is controlled at the level of the State Institute of Standardization and Control under Rospotrebnadzor of the Russian Federation. Numerous studies show that after vaccination, the incidence of influenza is reduced by 3-4 times compared to the control group, and due to polyoxidonium, resistance to other respiratory infections increases. For the creation of the vaccine, the team of authors was awarded the State Prize of the Russian Federation in 2002 [5, 6].
In risk groups (children, people over 50 years of age, patients with concomitant diseases, immunodeficiencies, allergic diseases, in which more severe infections, complications and deaths are observed), according to WHO recommendations, vaccination is carried out with subunit vaccines. Older adults are at high risk of serious complications, including secondary bacterial pneumonia, exacerbation of underlying chronic diseases, leading to hospitalization and increased mortality. Inactivated influenza vaccines have been used for more than 50 years; they are safe and tested on millions of people [7, 8]. Vaccination reduces the number of deaths, development and severity of complications [9–11, 13–15]. In children with allergic diseases, the Influvac vaccine has been shown to be effective [12]. To prevent epidemics, it is necessary to vaccinate the largest number of people. Given the large number of viruses that can cause acute respiratory infections, the incidence of influenza after vaccination should be confirmed serologically. In a randomized controlled trial (Netherlands), after vaccination of 1838 people over 60 years of age, serologically confirmed influenza cases accounted for 58% of acute respiratory infections [7]. The results of a meta-analysis show that vaccination against influenza among older people in various countries can reduce the number of hospitalizations by an average of 33%, including those associated with pneumonia and influenza by 27–38%, and overall mortality by 50% [ 11, 13].
Despite advances in prevention, vaccination coverage remains limited [24, 25]. In the United States in 1997, less than 30% of those vaccinated under the age of 65 were vaccinated; now, according to WHO, the number of vaccinated people is increasing. Full compliance of the vaccine structure with circulating strains is not always observed; accordingly, the protective effect of the vaccine is 70–90% [25], in risk groups, in the elderly, and in patients with immunodeficiency, the effectiveness decreases to 30–40% [26, 27].
Measures to prevent a pandemic must be taken in advance, because if it develops, the consumption of medications will increase significantly, the need for initial consultations, the number of visits to the doctor, hospitalizations, and complications will increase. According to WHO forecasts, during a pandemic, in a short period of time the number of visits to clinics will reach 233 million, hospital admissions – 5.2 million, deaths – 7.4 million people [28]. It is assumed that the most pronounced manifestations of the pandemic will be observed in underdeveloped countries due to insufficiently organized work of the health care system. On the other hand, there will be a shortage of vaccine and staff, which will lead to disruptions in the work of healthcare institutions, public transport, and law enforcement agencies. Without the development of a pandemic in the United States, from 10 thousand to 40 thousand people die annually from influenza, while in the last 60 years there has been no decrease in mortality rates from influenza pneumonia [29].
In Russia, from 27 million to 41 million patients with acute respiratory infections are registered annually, 95% of which are caused by viruses. In 2002, flu vaccinations in Russia accounted for 10–12%; in the 2013–2014 epidemic season. – 27.8% of the population [3]. According to the order of the Ministry of Health of the Russian Federation No. 125n dated March 21, 2014 “On approval of the national calendar of preventive vaccinations and the calendar of preventive vaccinations for epidemic indications,” the risk group includes: children from 6 months; students in grades 1–11 studying in professional educational organizations and educational organizations of higher education; adults working in certain professions and positions (employees of medical and educational organizations, transport, public utilities); pregnant women; adults over 60 years of age; persons subject to conscription for military service; persons with chronic diseases, including lung diseases, cardiovascular diseases, metabolic disorders and obesity [18].
Until the end of the 1970s. In Russia, vaccination against influenza was carried out with a live attenuated vaccine. Vaccination in risk groups with a live attenuated vaccine is impossible, which is associated with reactogenicity and the possibility of developing influenza in a weakened group of patients. In recent years, IV generation vaccines, virosomal vaccines, into which membrane antigens of the influenza virus are introduced, have been actively introduced into practical healthcare, which makes it possible to increase their immunogenicity and leads to the activation of cellular immunity, increases the titer and increases the duration of circulation of protective antibodies [17].
The new domestic inactivated split vaccine Ultrix® was obtained by treating influenza viruses with the detergent β-octyl glycoside and contains highly active pseudoviral particles in the form of virosomes, surface and internal antigens of influenza viruses A (H1N1 - 15 μg, H3N2 - 15 μg) and B ( 15 mcg). Administration of the vaccine does not lead to an increase in the level of immunoglobulin (Ig) E, which indicates its safety in allergic diseases [19]. Clinical trials have shown the vaccine to be safe in children over 6 years of age, adults 18–60 years of age, and adults over 60 years of age. The immunogenicity of the vaccine in terms of the level of seroconversion of antibodies to the influenza virus A (H1N1) is 94%, A (H3N2) is 86%, B is 90%. The composition of vaccine antigens changes according to WHO recommendations. After 6 months after vaccination, the protective titer of antibodies to influenza virus A (H1N1) remained in 81.3%, A (H3N2) in 61.5%, B in 47.3% of vaccinated people, while no significant decrease in antibody titer was observed. When vaccinating children, no pronounced reactogenicity was observed; the immunogenicity of the vaccine in terms of antibody seroconversion to influenza virus A (H1N1) was 70%, A (H3N2) – 50%, B – 70%. After vaccination, no pronounced general and local reactions were observed [3]. The effectiveness of vaccination was assessed in a clinical study in October–November 2013 among 5,743 residents of 7 regions of the Russian Federation, 325 of whom were over 60 years of age. A local reaction was observed in isolated cases, and no therapeutic intervention was required [3].
The results of vaccination against influenza in 9 schools in Podolsk, Moscow region showed a good effect, the incidence of influenza decreased by 4.7 times, and other acute respiratory viral infections by 1.4 times [20, 21]. According to V.K. Tatochenko, in whole-virion and split-virion influenza vaccines, the RNA of the virus can be preserved, which can lead to increased interferon synthesis and antiviral protection against other viruses. A study of the possibility of preventing other acute respiratory viral infections after vaccination with the Ultrix® vaccine was carried out among 594 medical workers, 1,389 people from closed organized groups in the Kaluga region, 1,000 residents of Timashevsk, Krasnodar Territory. Among unvaccinated healthcare workers, the incidence of influenza was 2.8 times higher compared to vaccinated workers. In closed groups, the incidence of influenza in those unvaccinated was 47 times higher compared to those vaccinated with the Ultrix® vaccine. The incidence of influenza in vaccinated residents of Timashevsk was 2.4 times lower than in the control group. A complicated course of ARVI in vaccinated people, if the patients became ill, was registered in 9.1% of cases, in the control group – in 36% of cases [3].
I. Feldblum et al. An open prospective randomized study was conducted including 1008 adults from 18 to 63 years of age, 504 of them were vaccinated with the Ultrix® vaccine, 504 people were not vaccinated (control group). Within 6 months. prospectively monitored the group. The immunogenicity of the vaccine was assessed by the level of seroconversion (the proportion of people whose antiviral antibody titer increased 4 times) and seroprotection (the proportion of people whose antibody titer was more than 1:40). Observation in the post-vaccination period revealed weak local reactions in 0.8% of vaccinated people, weak general reactions in 2.8%, combined reactions in 0.4% of vaccinated people. Follow-up for 6 months. did not reveal significant changes in the biochemical parameters of blood and urine (indicators of bilirubin, creatinine, urea, liver enzymes; in the urine there was no increase in the level of protein and leukocytes). Total IgE at the beginning of the study was 50.14 IU/ml; 180 days after vaccination it remained within the normal range – 88.3 IU/ml (the difference is significant). An increase in IgE levels may be associated with increased environmental and anthropogenic load in a large city (Perm), and the predominance of T-helper type 2 immune response. The immunogenicity of the vaccine in terms of seroconversion of antibodies to influenza virus A (H1N1) was 66.7%, A (H3N2) - 53.5%, B - 46.5%. The seroconversion factor to influenza virus A (H1N1) was 5.18, A (H3N2) – 3.94, B – 3.55. Seroprotection was observed against influenza virus A (H1N1) in 98%, A (H3N2) in 76.8%, and B in 70.7% of those vaccinated. After vaccination, 31 cases of ARVI were diagnosed in vaccinated people, and 32 cases of ARVI were diagnosed in the control group. The incidence rate among vaccinated people was 61.5 per 1 thousand people, in the control group - 85.3 per 1 thousand people (the difference is significant). In the vaccinated group, the diagnosis of influenza was not confirmed; in the control group, it was confirmed in 5 people (the duration of the disease was 7.6 days (moderate form). Thus, the protection coefficient was 100% [22].
I. Nikonorov et al. A comparative study of the Vaxigrip vaccine and the new virosomal vaccine Ultrix® was conducted during 2007, 2008 and 2010. on the basis of the Institute of Influenza and at the Perm State Medical Academy named after. E.A. Wagner in 2011 in accordance with the national standard. The study included 1,286 people, including 78 children, 40 adults over 60 years of age, and 1,208 adults under 60 years of age. Ultrix® was used in 2 dosages: 1) 35 mcg – HA antigens of influenza A viruses (H1N1 – 10 mcg, H3N2 – 10 mcg) and B (15 mcg); 2) 45 µg – HA antigens of influenza A viruses (H1N1 – 15 µg, H3N2 – 15 µg) and B (15 µg). The composition of the Vaxigrip vaccine is 45 mcg - HA antigens of influenza A viruses (H1N1 - 15 mcg, H3N2 - 15 mcg) and B (15 mcg). The results of observation and examination of patients by a therapist and otolaryngologist were recorded in individual registration cards. When vaccinating 150 clinically healthy volunteers aged 18–60 years, weak systemic reactions were observed in 8% with a dose of Ultrix® vaccine 35 mcg, in 12% with a dose of Ultrix® vaccine 45 mcg and in 8% with vaccination with Vaxigrip at a dose of 45 mcg . The IgE level at the first examination before vaccination exceeded the norm in 28% of cases with a dose of Ultrix® vaccine 35 mcg, in 14% of cases with a dose of Ultrix® vaccine 45 mcg and in 16% of cases before vaccination with Vaxigrip. The volunteers were not diagnosed with allergic diseases. Persons with allergic diseases were not vaccinated due to exclusion criteria. The immunogenicity of the Ultrix® 35 mcg vaccine in terms of seroconversion of antibodies to influenza virus A (H1N1) was 94%, A (H3N2) - 86%, B - 90%. The seroconversion factor to influenza virus A (H1N1) was 18, A (H3N2) – 8.6, B – 10.4. Seroprotection was observed against influenza virus A (H1N1) in 94%, A (H3N2) in 90%, and B in 78% of vaccinated people [29]. The immunogenicity of the Ultrix® 45 mcg vaccine was similar in terms of the level of seroconversion of antibodies to influenza virus A (H1N1) - 94%, A (H3N2) - 86%, B - 90%. The seroconversion factor to influenza virus A (H1N1) was 19.4, A (H3N2) – 9.4, B – 6.9. Seroprotection was observed against influenza virus A (H1N1) in 86%, A (H3N2) in 90%, B in 78% of those vaccinated. The immunogenicity of the Vaxigrip 45 mcg vaccine in terms of the level of seroconversion of antibodies to the influenza virus A (H1N1) was 98%, A (H3N2) - 94%, B - 88%. The seroconversion factor to influenza virus A (H1N1) reached 26.7, A (H3N2) – 9.7, B – 14.7. Seroprotection was observed against influenza virus A (H1N1) in 100%, A (H3N2) in 94%, B in 86% of vaccinated people [29]. At the second stage, the possibility of vaccinating 40 people over 60 years of age was studied. Data on the feasibility of vaccination in older people remain controversial [30]. 20 people were vaccinated with the Ultrix® 45 mcg vaccine and 20 people with Vaxigrip 45 mcg. Mild systemic reactions were noted in 15% of patients in each group. After vaccination, the level of total IgE remained within normal limits. In the elderly, the immunogenicity of the Ultrix® 45 mcg vaccine was similar in terms of the level of seroconversion of antibodies to influenza virus A (H1N1) - 80%, A (H3N2) - 85%, B - 65%. The seroconversion factor for influenza virus A (H1N1) was 9.5, A (H3N2) - 12.1, B - 28.3. Seroprotection was observed against influenza virus A (H1N1) in 70%, A (H3N2) in 90%, and B in 50% of those vaccinated. The immunogenicity of the Vaxigrip 45 mcg vaccine in the elderly in terms of the level of seroconversion of antibodies to the influenza virus A (H1N1) was 95%, A (H3N2) - 90%, B - 80%. The seroconversion factor to influenza virus A (H1N1) reached 21.9, A (H3N2) – 12.6, B – 7.5. Seroprotection was observed against influenza virus A (H1N1) in 95%, A (H3N2) in 90%, B in 80% of vaccinated people [29]. At the next stage, 36 children aged 12–18 years were vaccinated, and in 2010–2011. 42 children aged 6–12 years were vaccinated. Children were randomly divided into groups receiving Ultrix® 45 mcg and Vaxigrip 45 mcg. No increase in total IgE was observed in children during vaccination. No allergic diseases were reported, but 55% of children aged 6–12 years had elevated IgE levels and decreased during follow-up after vaccination.
The immunogenicity of the Ultrix® 45 mcg vaccine in children 12–18 years old was 70% in terms of seroconversion of antibodies to influenza virus A (H1N1), 50% for A (H3N2), and 70% for influenza virus. The seroconversion factor for influenza virus A (H1N1) was 6.5, A (H3N2) – 2.7, B – 4. Seroprotection was observed for influenza virus A (H1N1) in 90%, A (H3N2) – in 80%, B – in 85% of vaccinated people. The results are comparable to the results of vaccination with Vaxigrip 45 mcg [29]. Thus, studies have shown good safety, immunogenicity and low reactogenicity of the new domestic influenza virosomal vaccine Ultrix® in children over 6 years of age and adults, including people over 60 years of age.
Literature 1. Influenza and other acute respiratory viral infections / ed. prof. V.P. Maly and prof. M.A. Andreychina M.: GEOTAR-Media, 2013. 319 p. 2. Gendon Yu.Z. Analysis of influenza activity during the 2003/2004 epidemic season. // Vaccine prevention news. Vaccination. 2004. No. 3. P. 6. 3. Selkova E.P., Grenkova T.A., Gudova N.V. and others. Epidemiological significance of influenza vaccine prevention. Domestic influenza vaccine of the latest generation // Epidemiol. and infectious diseases. Current issues. 2014. No. 4. pp. 43–51. 4. WHO. Guidelines for pharmacological management of pandemic (H1N1) 2009 influenza and other influenza viruses. Publication date: August 20, 2009. 5. Khaitov R.M., Nekrasov A.V., Bektemirov T.A. Allergy, asthma and clinical immunology. 1999. No. 9. pp. 7–9. 6. Bektemirov T.A., Gorbunov M.A., Elshina G.A. Prospects for the use of polyoxidonium with vaccines against viral hepatitis and influenza // Allergy, asthma and clinical immunology. 2001. No. 1.S. 63–65. 7. World Health Organization. Influenza vaccines // Wkly Epidemiol Rec. 2000. Vol. 75. R. 281–288. 8. World Health Organization. Influenza vaccines // Wkly Epidemiol Rec. 2002. Vol. 77. R. 229. 9. Uchaikin V.F., Shamsheva O.V. Vaccine prevention. Present and future. M.: GEOTAR-Med, 2001. 399 p. 10. Palach A.M. Tens years of experience with a subunit influenza vaccine // Europ.J.of Clin.Research. 1992. Vol. 3. R. 117–138. 11. Palach A.M., de Bruijn IAJ Nauta. Influenza immunization // J. of Clin. Research. 1999. Vol. 2. R. 111–139. 12. Markova T.P., Chuvirov D.G. The use of the Influvac vaccine for the prevention of influenza in children with allergic diseases // Ross. Bulletin of Perinatology and Pediatrics. 2001. No. 6. P. 53–54. 13. Nichol KL Wuomema J, von Sternberg T. Benefits of influenza vaccination for low-, intermediate-, and high-risk senior citizens // Arch Intern Med. 1998. Vol.158. R. 1769–1776. 14. Uphoff H., Cohen JM, Fleming D., Noone A. Harmonization of national influenza surveillance morbidity data from EISS: a simple index // Euro Surveill. 2003 Jul. Vol. 8 (7). R. 156–164. 15. Aymard M. Hospices Civils de Lyon, France. Presentation at a symposium entitled. Meeting the challenge of influenza., European Respiratory Society 1998 Annual Congress, Geneva, Switzerland. 16. Letter of Rospotrebnadzor dated June 24, 2013 No. 01/7080-13-32 “On the results of the spread of influenza and ARVI in the world and the Russian Federation during the 2012-2013 epidemic season.” and forecast for the 2013-2014 epidemic season.” 17. Bruljn IA, Nauta J., Gerez L. Virosomal influenza vaccine: a saving and effective influenza vaccine with high efficacy in elderly and subjects with low pre-vaccination titers // Virus Research. 2004. Vol. 103. P. 139–145. 18. Order of the Ministry of Health of Russia dated March 21, 2014 No. 125n “On approval of the national calendar of preventive vaccinations and the calendar of preventive vaccinations for epidemic indications.” 19. Zverev V.V., Erofeeva M.K., Maksakova M.L. and others. Development and introduction into healthcare practice of the Russian Federation of a new domestic split virosomal vaccine against influenza // Attending physician. 2008. No. 9. pp. 68–70. 20. Briko N.I. Vaccination is a decisive measure for the prevention of influenza // Attending physician. 2011. No. 8. pp. 90–93. 21. Khaitov R.M., Nekrasov A.V., Lytkina I.N. and others. On the influence of vaccination on the incidence of influenza and ARVI // Vaccinal prevention of influenza. Vaccination. 2001. No. 5 (7). pp. 27–34. 22. Feldblyum I., Polushkina A., Vorobyova I. Immunization of adults 18-60 years old with the domestic influenza virosomal vaccine Ultrix // Doctor. 2014. No. 9. pp. 54–56. 23. Nikanorov I., Maksakova V., Feldblyum I. et al. Domestic drug of the latest generation for the prevention of influenza // Doctor. 2014. No. 3. P. 1–6. 24. Murphy BR, Webster RG Orthomyxoviruses. In: Fields BN et al. editors. Fields virology, 3rd Edn. Philadelphia, USA: Lippincott–Raven, 1996, pp. 1397–445. 25. Palache AM Influenza vaccines. A reappraisal of their use // Drugs. 1997. Vol. 54. R. 841–856. 26. Arden NH, Patriarca PA, Kendal AP Experiences in the use and efficacy of inactivated influenza vaccine in nursing homes. In: Kendal AP, Patriarca PA, editors. Options for the Control of Influenza. New York; Alan R. Liss Inc., 1986, pp. 155–168. 27. Patriarca PA, Weber JA, Parker RA et al. Efficacy of influenza vaccine in nursing homes: reduction in illness and complications during an influenza A (H3N2) epidemic // JAMA. 1985. Vol. 253. R. 1136–1139. 28. WHO checklist for influenza pandemic preparedness planning. Geneva, World Health Organization, 2005. 29. Center for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) // MMWR. 2000. Vol. 49. R. 1–38. 30. Lang P., Mendes A., Socquet J. et al. Effectiveness of influenza vaccine in aging and older adults: comprehensive analysis of the evidence // Clin. Interv. Aging. 2012. Vol. 7. P. 55–64.
What is included in the Ultrix Quadri vaccine?
The exact composition of antigens, like other influenza vaccines, is updated annually in accordance with WHO recommendations. The dosage of each is at least 15 mcg, which is recommended by WHO. In addition to them directly, the drug contains only 3 components: polysorbate 80, widely used in the food industry as an emulsifier, octoxynol 10 as a stabilizer and phosphate buffer solution. Ultrix Quadri does not contain additional immune response enhancers, for which some other domestic drugs have been repeatedly criticized.
METHOD OF APPLICATION AND DOSAGE
Vaccination is carried out annually in the autumn-winter period. Vaccination is possible at the beginning of an epidemic rise in the incidence of influenza.
The vaccine is administered intramuscularly in a dose of 0.5 ml once in the area of the deltoid muscle (the upper third of the outer surface of the shoulder).
The drug is not suitable for use in syringes/vials with damaged integrity or markings, if the physical properties (color, transparency) have changed, if there are foreign particles in the solution, if the expiration date has expired, or if the requirements for storage conditions are violated.
Opening of syringes/vials and vaccination is carried out in strict compliance with the rules of asepsis and antiseptics. The drug cannot be stored in opened syringes/vials.
What contraindications exist for Ultrix Quadri?
Vaccination should be postponed if the child is ill in the acute stage, for a period of 2 to 4 weeks - it is better to consult with your personal pediatrician about the necessary break. For mild respiratory or intestinal infections, you need to wait until the temperature drops, then, as a rule, you can get vaccinated. The vaccine should not be given if the child has had a strong reaction to previous flu vaccinations (no matter what kind of vaccine) or is allergic to any of the components, including chicken protein.
Patients who have recovered from covid-19 can be vaccinated against influenza 1 month after recovery.
Sources:
- Instructions for use of the Ultrix Quadri vaccine
- Rostec’s vaccine against four strains of influenza “Ultrix Quadri” has been approved for all ages.//Rostec, 09/21/2021
- Creator of the new Russian flu vaccine Anton Katlinsky: We vaccinated Sergei Chemezov and Veronika Skvortsova.//Snob, 10/15/2019
MAKE AN APPOINTMENT PRICES
PRECAUTIONARY MEASURES
- Do not administer intravenously.
- Before vaccination, those vaccinated must be examined by a doctor (paramedic) with mandatory thermometry. If the body temperature is above 37C, vaccination is not carried out.
- Rooms where vaccination is carried out must be equipped with anti-shock therapy. The vaccinated person should be under the supervision of a health care worker for 30 minutes after vaccination.
Use during pregnancy and breastfeeding
Experience with the use of inactivated influenza vaccines shows that vaccination of women during breastfeeding does not have a toxic effect on the child .
The final decision to vaccinate pregnant and breastfeeding women should be made individually by a physician, taking into account the risk of contracting influenza and possible complications caused by influenza.
The safest period for vaccination of pregnant women is the second and third trimesters of pregnancy.
INTERACTIONS WITH OTHER MEDICINES
The vaccine can be used simultaneously with inactivated and live vaccines of the National Preventive Vaccination Calendar of the Russian Federation (with the exception of tuberculosis vaccines and inactivated vaccines of the preventive vaccination calendar for epidemiological indications (except for rabies). In this case, contraindications to each of the vaccines used must be taken into account; the drugs should be administered in different parts of the body with different syringes.
The vaccine can be administered against the background of basic therapy for the underlying disease. Vaccination of patients who have received immunosuppressive therapy (glucocorticosteroids, cytotoxic drugs, radiotherapy) may be less effective.