Medical Internet conferences
Relevance. Tuberculosis in the modern period is an urgent medical and social problem, which is due to the high level of morbidity, disability and mortality from this disease [1]. In recent years, an increase in drug resistance (DR) of Mycobacterium tuberculosis (MBT) has been recorded, which is a sign of deepening epidemic troubles [1, 5]. A particularly big problem is multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis. According to Morozova T.I. (2014) in the Saratov region, the MDR level is 15.0%; as of January 1, 2015, 140 tuberculosis patients with XDR MBT were registered. The effectiveness of treatment in this group of patients is about 48% [4]. A significant role in increasing the effectiveness of chemotherapy in people with MDR and XDR tuberculosis belongs to the introduction of new anti-tuberculosis drugs into practice. At the end of 2013, Bedaquiline (Sirturo, Janssen Therapeutics, a division of Janssen Products LP) was approved for the treatment of MDR tuberculosis in adults. In Federal regulations, the drug is recommended for inclusion in the treatment regimen for tuberculosis with XDR MBT [2]. There are 2 studies involving 440 patients with multidrug-resistant forms of tuberculosis, defined as MDR tuberculosis [7]. According to both studies (both placebo-controlled and open-label), the use of Bedaquiline led to the cessation of bacterial excretion by week 24 of treatment in 79% of patients, while there are significant aspects that relate to the safety of the drug, namely an increased risk of prolongation of the QT interval and hepatotoxicity. Currently, there is a need to obtain more data on the clinical course of drug-resistant tuberculosis with the use of Bedaquiline.
Purpose of the study. Using a clinical example, demonstrate the effectiveness of using the new anti-tuberculosis drug Bedaquiline in the treatment of drug-resistant tuberculosis.
Materials and methods. Treatment was carried out in accordance with current regulatory documents [3, 6]. The assessment of the dynamics of a specific process was carried out within the time limits regulated by law (examination of sputum for the presence of bacterial excretion by bacterioscopy and culture, blood, urine, X-ray examination).
Results.
Patient T., 32 years old, was admitted for treatment to the regional clinical tuberculosis dispensary (RCTD) in Saratov on October 10, 2013.
History: Resident of Saratov, disabled group 2 with tuberculosis, not married, no children. Secondary education. Bad habits: smokes a pack of cigarettes a day since the age of 18. Drinks alcohol in moderation. She has been observed by a phthisiatrician since August 2011 with a diagnosis of infiltrative tuberculosis of the upper lobe of the right lung in the decay phase of MTB(+). Anti-tuberculosis therapy was carried out with drugs of the main series - isoniazid (H), rifampicin (R), pyrazinamide (Z), ethambutol (E). During 2 months of chemotherapy (CT), clinical and radiological dynamics of a specific process were not recorded; when sputum was examined by culture for the sensitivity of MBT to chemotherapy, resistance to H, R, streptomycin (S) was revealed - MDR MBT. The patient felt satisfactory, did not comply with the treatment regimen, and “took a break” from observation. And only in October 2013 he was again involved in inpatient treatment at OKPTD.
At the time of admission, the patient complained of cough with mucopurulent sputum, moderate weakness, shortness of breath on exertion, and sweating at night.
Objective status on admission: Condition is relatively satisfactory. Reduced nutrition. Enlarged axillary lymph nodes up to 1 cm in diameter were detected, mobile, painless, not fused with surrounding tissues. The supraclavicular fossae are pronounced. From the respiratory organs: percussion pulmonary sound with a boxy tint at the apex of the right lung, auscultation at the apex of the right lung amphoric breathing, single medium-bubble moist rales. NPV 20 per minute. No visible pathology in other organs and systems.
X-ray: the upper lobe of the right lung is infiltrated, reduced in volume, the interlobar pleura is retracted, against the background of massive infiltration, an oval-shaped cavity of 4.0×3.5 cm is determined in the upper lobe of the right lung, focal shadows are visible in the underlying lung tissue. Conclusion: Fibrous-cavernous tuberculosis of the right lung in the phase of infiltration and seeding. Atelectasis of the upper lobe of the right lung.
In the general blood test: accelerated ESR up to 45 mm/hour, other parameters are within normal limits. FVD study: DN1 according to the restrictive type.
Taking into account the data of the anamnesis, objective examination, instrumental and laboratory research methods, a diagnosis was made: Fibrous-cavernous tuberculosis of the right lung in the phase of infiltration and contamination of MTB (+) MDR (HRS), atelectasis of the upper lobe of the right lung. In accordance with modern approaches, treatment was prescribed using 4 chemotherapy regimens: Z, E, prothionamide (Pt), levofloxacin (Lfx), aminosalicylic acid (PAS), capreomycin (Cm).
After 2 months (December 2013) of complex chemotherapy, negative clinical and radiological dynamics were noted. Symptoms of intoxication persist. When studying FVD - DN2, mixed type with a predominance of restrictive changes. X-ray revealed progression of the process due to the appearance of “fresh” foci and infiltration with decay in S6 on the right. Abundant bacterial excretion during sputum examination by the Ziehl-Neelsen method and culture continues. In December 2013, the results of sputum culture for the sensitivity of MBT from October 2013 were obtained and it was established that the MBT DR spectrum had expanded to kanamycin (K), ofloxacin (Ofl), Cm - XDR. The treatment regimen has been adjusted.
Against the background of the therapy, by February 2014, a decrease in symptoms of intoxication was observed, while a productive cough and abundant bacterial excretion in sputum remained by all methods. The hemogram shows a slight decrease in ESR to 29 mm/h. X-rays revealed a 2-fold decrease in infiltration in S6 on the right and a more than 2-fold decrease in the decay cavity in S6 on the right. Considering the positive clinical and radiological dynamics of the process, the patient’s treatment was continued with the same set of medications. However, in April 2014, radiography again noted the progression of a specific process due to an increase in one of the decay cavities in the right lung, while the clinical picture and hemogram parameters remained stable. In June 2014, the result of the MBT drug sensitivity test from April 2014 was received, which noted a further expansion of the MBT DR spectrum. X-ray, when compared with data from April 2014, negative dynamics due to an increase in the size of one of the cavities on the right in S6. The upper lobe of the right lung is reduced in volume due to fibroatelectasis, against the background of which a cavity of 4.5×4.0 cm remains. In the S6 projection, against the background of fibrosis of the decay cavity, 2.7×2.0 cm, 2.0×1.3 cm, 2.5×2.5 cm with wall thickness up to 0.3 cm. There are foci in the adjacent lung tissue.
Taking into account the steady progression of tuberculosis, the persistence of bacterial excretion in sputum by all methods, and XDR MBT (HRSCOflCapE), it was decided to add 3rd line drugs to the treatment. Thus, therapy was carried out with the following set of drugs: ZPtLfxPas, bedaquiline (Bq) 400 mg 1 time / day for the first 14 days, then 200 mg 3 times / week per os + clarithromycin (Clr) 500 mg 1 time / day per os.
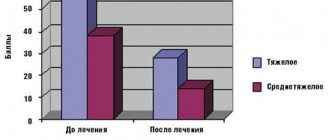
Against the background of changing the chemotherapy regimen, clinical improvement was observed within a month: a significant decrease in symptoms of intoxication, cough, increased appetite, and improved well-being. In July, for the first time in several months, a negative result of a sputum examination for MBT was obtained using microscopy, which was subsequently confirmed by repeated examination of sputum using the Ziehl-Neelsen method. In the general blood test, ESR decreased from 26 to 15 mm/hour, leukocytosis from 11.7 to 7.8×109/l. External respiration function was restored according to spirography data (Table).
During an X-ray examination in August 2014, after 2 months of treatment with Bedaquiline, a significant 2-fold decrease in infiltration and decay cavities in S1,2,6 of the right lung was recorded.
The tolerability of the drugs in the treatment regimen with Bedaquiline is satisfactory. To dynamically monitor the QT interval, the patient underwent an ECG examination at the time of admission, 2, 4, 8 weeks from the start of treatment. No deviations were found.
Due to the stabilization of the tuberculosis process, the patient with a diagnosis of fibrous cavernous tuberculosis of the right lung MBT (+) XDR (H,S,K,R,Ofl,Cap,E), fibroatelectasis of the upper lobe of the right lung was sent for surgical treatment to a clinical hospital phthisiopulmonology MMA named after. THEM. Sechenov, where on September 19, 2014, an operation was performed: video-assisted (VATS) upper lobectomy with resection of S6,10 of the right lung. Histologically: fibrous-cavernous tuberculosis of the right lung. On 10.10.14, VATS 3-rib thoracoplasty on the right was performed.
Currently, the patient is receiving therapy in a sanatorium. The clinical and radiological picture was stable for 4 months.
Conclusion.
Thus, the presented clinical observation demonstrates that the addition of the drug “Bedaquiline” to the anti-tuberculosis therapy regimen promotes clinical improvement and cessation of bacterial excretion in a fairly short time, leads to the involution of specific changes in the lung tissue, which makes it possible to achieve stabilization of the process in tuberculosis patients with confirmed XDR pathogen , subsequently use surgical intervention and increases the patients’ chances of a positive vital and social prognosis.
Sirturo tablets 100 mg, 188 pcs.
Hypersensitivity to the active substance or any of the excipients. Interactions with other drugs and other types of interactions The in vivo effects of bedaquiline have not been fully determined. CYP3A4 is the main CYP isoenzyme. involved in the metabolism of bedaquiline and the formation of the metabolite .V-monodesmethyl (M2) in vitro. The excretion of bedaquiline in urine is insignificant. Bedaquiline and M2 are not substrates or inhibitors of P glycoprotein.
CYP3A4 inducers
Exposure to bedaquiline may be decreased when used concomitantly with CYP3A4 inducers.
In a single-dose interaction study of bedaquiline and once-daily rifampicin (a strong inducer) in healthy volunteers, bedaquiline exposure (AUC) was reduced by 52% [90% CI (-57, -46)]. Due to the potential for decreased therapeutic effect of bedaquiline due to decreased systemic exposure, concomitant use of bedaquiline and moderate or strong CYP3A4 inducers (e.g., efavirenz, etravirna, rifamycins including rifampicin, rifapentine and rifabutin, carbamazepine, phenytoin, St. John's wort (Hypericum perforatum)) should be avoided. for systemic use.
CYP3A4 inhibitors
Exposure to bedaquiline may be increased when administered concomitantly with CYP3A4 inhibitors.
Short-term concomitant use of bedaquiline and ketoconazole (which is a strong CYP3A4 inhibitor) in healthy volunteers increased bedaquiline exposure (AUC) by 22% [90% CI (12, 32)]. A more pronounced effect on bedaquiline may be observed with long-term concomitant use of ketoconazole or other CYP3A4 inhibitors.
There are no safety data from multiple-dose studies of bedaquiline that used a dose higher than that indicated. Due to the potential risk of adverse reactions due to increased systemic exposure, prolonged concomitant use of bedaquiline and systemic moderate or strong inhibitors (eg, ciprofloxacin, erythromycin, fluconazole, clarithromycin, ketoconazole, ritonavir) for more than 14 consecutive days should be avoided. .. If simultaneous use is required, it is recommended to conduct more frequent ECG monitoring and monitoring of transaminase levels (see section “Peculiarities of use”).
Other anti-tuberculosis drugs
Short-term concomitant use of bedaquiline with isoniazid/pyrazinamide in healthy volunteers did not result in clinically significant changes in the exposure (AUC) of bedaquiline, isoniazid, or pyrazinamide. When used concomitantly with bedaquiline, no dosage adjustment of isoniazid or pyrazinamide is necessary.
In a placebo-controlled clinical trial in patients with tuberculosis caused by multidrug-resistant Mycobacterium tuberculosis, no significant effects of concomitant use of bedaquiline on the pharmacokinetics of ethambutol, kanamycin, pyrazinamide, ofloxacin or cycloserine were observed.
Antiretroviral drugs
In an interaction study of single-dose bedaquiline and multiple doses of lopinavir/ritonavir, bedaquiline exposure (AUC) was increased by 22% [90% CI (11, 34)]. A more pronounced effect on bedaquiline plasma levels may be observed with long-term concomitant use with lopinavir/ritonavir. Published data from patients treated with bedaquiline as part of resistant TB therapy and lopinavir/riponavir-based APT showed that bedaquiline exposure (AUC) over 48 hours increased approximately 2-fold. This increase was significantly associated with ritonavir. If the benefit outweighs the risk. Sirturo can be used with caution when used concomitantly with lopinavir/ritonavir. When used concomitantly with other ritonavir-boosted HIV protease inhibitors, bedaquiline plasma levels are expected to increase. It should be noted that no changes in bedaquiline dosage are recommended when used concomitantly with lopinavir/ritonavir or other ritonavir-boosted HIV protease inhibitors. There are no data to support the need to reduce the dose of bedaquiline in these circumstances.
Concomitant use of a single dose of bedaquiline and multiple doses of nevirapine did not result in clinically significant changes in bedaquiline exposure. There are no clinical data regarding the simultaneous use of bedaquiline and antiretroviral drugs in patients coinfected with human immunodeficiency virus and multidrug-resistant Mycobacterium tuberculosis (see section "Peculiarities of use"). Efavirenz is a moderate inducer of CYP3A4 activity and its simultaneous use with bedaquiline may lead to a decrease in the effect of bedaquiline and loss of activity, and therefore their simultaneous use is not recommended.
Medicines that prolong the QT interval
Information about the potential for pharmacodynamic interaction between bedaquiline and drugs that prolong the QT interval is limited. In an interaction study between bedaquiline and ketoconazole, a greater effect on QTc was observed after multiple doses of bedaquiline and ketoconazole in combination than after multiple doses of either drug alone. An additive or synergistic effect of bedaquiline on QT prolongation when used concomitantly with other drugs that prolong the QT interval cannot be excluded; It is recommended to carry out monitoring more frequently (see section “Peculiarities of application”).
OT interval and simultaneous use of clofazimine
In the open-label Phase III trial, the mean increase in QTcF was greater in 17 patients concomitantly taking clofazimine at week 24 (mean change of 31.9 ms compared to comparator) than in patients not concomitantly taking clofazimine at week 24 (the average change was 12.3 ms compared to the comparison drug) (see section “Peculiarities of application”).
Children
Interaction studies were conducted in adult patients only.
Sirturo
Treatment should be carried out under the direct supervision of a specialist.
Strains of M. tuberculosis isolated from a patient who has failed sputum conversion on therapy or has relapsed after completion of treatment should be tested for bedaquiline sensitivity (MIC).
Impact on mortality
In a randomized phase 2 clinical trial (C208. stage 2), an increase in mortality was noted in the drug group (10/79) compared with the placebo group (3/81). In all 5 cases of death from tuberculosis, sputum conversion was not achieved at the last examination. In other cases, the causes of death were alcohol poisoning, hepatitis/liver cirrhosis, septic shock, peritonitis, cerebrovascular accident, and a traffic accident. One death out of 10 occurred during the first 24 weeks of therapy. The remaining 9 cases of mortality in patients occurred after stopping the drug. The observed imbalance in mortality between the two groups is inexplicable. There was no significant correlation between mortality and sputum conversion, relapse, sensitivity data to other anti-TB drugs, HIV status, or disease severity.
During the clinical study, patients who died did not experience significant prolongation of the QTc interval or clinically significant arrhythmias.
In an open-label phase 2 study (C209), 16 of 233 (6.9%) patients died, 9 patients had tuberculosis as the cause of death, of which 8 patients were at risk of developing unfavorable dynamics or relapse of the process. In other cases, death was from other causes.
Effect on prolongation of the OT interval
In a controlled phase 2 study, a mean increase in QT corrected by Frederick's formula (QTcF) was observed starting at 1 week of therapy. The greatest increase in mean QTcF during 24 weeks of therapy was observed at week 18 and was 15.7 ms in the drug group compared to 6.2 ms in the placebo group. After finishing taking the drug (i.e. after the 24th week), the QTcF interval did not reach normal values in this group.
In an open-label phase 2 study (C 209), patients treated with other anti-TB drugs known to prolong the QTcF interval, including clofazimine, experienced an increase in QTcF interval proportional to the number of drugs in the treatment regimen.
In patients whose treatment regimen included only Sirturo, as a drug that affects the prolongation of the QTcF interval, the maximum increase in the interval was 23.7 ms and did not exceed 480 ms. In patients with at least two drugs in the treatment regimen with a side effect on the QTcF interval, a maximum increase in the interval of 30.7 ms was observed; an interval prolongation of more than 500 ms was observed in one patient.
Cases of the development of polymorphic ventricular tachycardia 'torsade de pointes' have not been recorded.
In clinical studies, there was no clear correlation of clinically significant prolongation of the QT interval or cardiac arrhythmias among patients with fatal outcome.
Before starting therapy and then monthly, an ECG study is necessary to dynamically monitor the QTcF interval.
Before starting therapy, it is necessary to assess the concentration of potassium, magnesium and calcium in the blood serum and adjust the values in case of deviation from normal values. Follow-up monitoring of electrolytes is recommended monthly.
Initiation of therapy is not recommended in patients with the following conditions, unless the benefits of the drug outweigh the potential risks:
- QT interval corrected according to the Frederick formula (QTcF) >450 ms (with confirmation using a repeat ECG study);
- decompensated heart failure;
- personal or family history of congenital prolongation of the QT interval or development of ari;
- bradyarrhythmia, including a history of it;
— electrolyte disturbances (hypocalcemia, hypomagnesemia, hypokalemia);
- hypothyroidism, including a history.
There are no data on the use of the drug in patients with ventricular arrhythmias and a history of myocardial infarction.
Therapy with any other drugs that prolong the QT interval should be discontinued if the patient develops clinically significant ventricular arrhythmia or a QT interval corrected by Frederick's formula (QTcF) > 500 ms (confirmed by repeat ECG testing). Frequent ECG monitoring should be performed until the QT interval returns to normal. In the event of an attack of short-term loss of consciousness, an ECG study is necessary to confirm the prolongation of the QT interval.
When co-using bedaquiline with drugs that cause prolongation of the QT interval (in particular with fluoroquinolone antibiotics, macrolides, clofazimine), caution should be exercised, since an additive or synergistic effect cannot be excluded, which can lead to a significant prolongation of the QT interval. If the combined use of such drugs with bedaquiline is necessary, clinical monitoring of the patient is recommended, including regular ECG monitoring.
Effect on liver function
During therapy (in combination with other anti-tuberculosis drugs) in clinical studies, more frequent adverse reactions from the liver were noted in comparison with a combination therapy regimen with anti-tuberculosis drugs without the addition of the drug. In this regard, monitoring of the patient’s clinical condition and a biochemical blood test should be carried out to determine the activity of liver enzymes (AST, ALT) and indicators of cholestasis (alkaline phosphatase, bilirubin level) before starting therapy, monthly during treatment and, if necessary, more often.
In patients taking the drug, if previously undocumented clinically significant changes in liver function parameters or further deterioration of liver function (assessed by AST, ALT and/or bilirubin levels) appear, as well as the presence of clinical symptoms (such as fatigue, anorexia, nausea, jaundice, dark urine, hepatomegaly) it is necessary to carefully monitor the patient and follow the algorithm for managing adverse reactions.
If the activity of aminotransferases (ALT, AST) exceeds the upper limit of normal by 5 times, then it is necessary to reconsider the treatment regimen and stop taking the drug and/or taking hepatotoxic drugs.
Patients of different ethnic groups do not require dose adjustment.
If adverse reactions from the central nervous system occur while using the drug, patients are advised to refrain from driving vehicles and engaging in other potentially hazardous activities that require increased concentration, speed of psychomotor and motor reactions.