The affordable price of the drug Dibikor belongs to the group of drugs that improve metabolism and energy supply to tissues. It is produced in the form of white round tablets of 250 mg. They are packaged in aluminum blisters. The main active ingredient in the medication is taurine.
Active ingredient
Taurine is a substance that is produced in the human body. It was discovered and described in 1827. With the help of studies, it has been proven that with its deficiency, malfunctions in the functioning of many internal organs are observed.
Taurine is important for proper liver function, participates in hematopoiesis and supports the functioning of the nervous system. Its importance cannot be overestimated in metabolism, in the processes of regulating blood pressure and digesting fats in the digestive system.
With a sufficient amount of taurine in the body, the immune system is strengthened and the general condition of the body is stabilized. This substance relieves fatigue and increases performance. The substance works as an antioxidant and after heavy physical exertion ensures rapid recovery of the body. It is difficult to overestimate the effect of taurine on eye health. It keeps the retina in good condition.
The main effects of taurine on the human body are as follows:
- In improving the functional state of the cardiovascular system.
- In the normalization of carbohydrate and lipid metabolism.
- In stabilizing the condition of the liver.
Indications and contraindications
You can buy the drug Dibicor, the instructions for use confirm this, for the treatment and prevention of various diseases. It is prescribed for diabetes mellitus types 1 and 2. This disease is today considered one of the main health problems of people in the modern world.
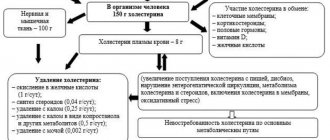
According to clinical studies, the drug regulates blood glucose levels. In addition, it leads to normal cholesterol and blood pressure levels. While taking dibicor, patient reviews confirm this, metabolic processes in the heart and liver improve. Thanks to this, it is possible to stabilize the condition and improve the quality of life of people suffering from diabetes.
Other indications for the drug are indicated in the instructions. First of all, it is cardiovascular failure. The drug reduces congestion and intracardiac pressure, stabilizing the condition. The drug also reduces the intensity of negative symptoms during intoxication caused by cardiac glycosides.
Dibicor is prescribed during antifungal therapy. Thanks to the drug, it is possible to protect the liver. The drug is also indicated for lowering cholesterol. This is due to the fact that taurine is contained in bile acids, which are responsible for removing cholesterol from the body. Therefore, deficiency of the substance impairs cholesterol metabolism. In addition, when taking the drug, the risks of bile flow disorders and stone formation are minimized.
An absolute contraindication is intolerance to the active substance and other components in the medication. It is prohibited to use Dibicor before the age of 18, since appropriate studies have not been conducted on the effect of the drug on the maturing body. You should stop using the drug during pregnancy, since no studies have been conducted on the effect of the drug on the fetus. For the same reason, the drug should not be used during lactation.
Results and its discussion
In both groups, during NASH therapy, there was an improvement in the general condition of the patients: weakness decreased, performance increased, and dyspeptic and pain syndromes were relieved. During treatment, no side effects of the drug Dibikor were identified.
. Dynamics of biochemical blood test parameters in patients of group 1 (without Dibikor).
. Dynamics of biochemical blood test parameters in patients of group 2 (who took Dibicor).
When analyzing the dynamics of biochemical blood parameters in both groups (Tables 1 and 2), normalization of indicators of activity (cytolysis) - transaminases was noted, but only in the Dibikor group (Table 2) positive changes in the lipid spectrum were revealed: the levels of TC, TG and AI (p < 0.05); the indicators of free bilirubin, fibrinogen, alkaline phosphatase, GGTP, and fasting glycemia significantly decreased (p < 0.05). In the same group (Table 3, p < 0.01), in contrast to group 1 (Table 3), a decrease in BMI was noted during the control study. The change in DBP was more pronounced, but not significant (Table 4). Subsequently, we calculated statistical criteria for the probability of alpha error. Quantitative assessment allows us to conclude that there is a low probability that the observed differences in the effectiveness of treatment regimens are due to chance.
. Dynamics of weight, BMI and WC/OB index.
. Dynamics of blood pressure.
Application
Self-medication with this drug is prohibited. It is important to exclude contraindications before starting to take the drug. The dosage of the drug is prescribed by the doctor individually, depending on the existing disease and the patient’s condition. To guarantee a positive result from taking dibicor, you need to take it for at least 3 months.
Tablets are taken before meals, at least 15-20 minutes. They need to be washed down with a small amount of water. According to the instructions, in order to stabilize the condition of cardiovascular failure, it is recommended to take 1-2 tablets 2 times a day. Depending on the body’s reaction and the results obtained, the dose of the drug can be changed by the doctor to decrease or increase.
In other cases, you can use the basic recommendations that are indicated in the instructions for use. It is important not to overdose the drug. Otherwise, the risks of negative side reactions of the body increase. As a rule, these are allergic manifestations on the skin.
Material and methods
The study, conducted at the gastroenterology department of ICH No. 7 of Novosibirsk, included 36 patients with NAFLD in the NASH stage of minimal biochemical activity (transaminase levels - from 1.5 to 2.5 norms) of both sexes aged from 18 to 65 years with a disease duration of at least a year. Exclusion criteria were: viral hepatitis B and C, alcohol history, concomitant pathology in the stage of subcompensation or decompensation, taking medications that could potentially lead to liver dysfunction; the doctor's lack of confidence in the patient's adherence to treatment.
The diagnosis of NAFLD is based on the presence of the “white liver” symptom on ultrasound examination (ultrasound) and signs of metabolic syndrome. NASH as a variant of NAFLD is established when the patient has cytolysis syndrome (hyperenzymemia, which cannot be explained by other reasons). The duration of observation was one month for each patient. The patients were divided into two groups. All of them received therapy, which included nutritional recommendations, essential phospholipids, silymarin, metformin, vitamins, and patients of the second group (n = 20) additionally took Dibicor 0.5 g 2 times a day 20 minutes before meals.
Before the start of the study and post-month treatment, all patients underwent an ECG, a biochemical blood test, which included quantitative determination of alanine (ALT) and aspartic (AST) transaminases, glycemic profile, alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGTP), free and bound bilirubin, total cholesterol (TC), high-density lipoproteins (HDL) and low-density lipoproteins (LDL), triglycerides (TG); The atherogenic index was calculated: AI = (TC-HDL)/HDL. In all patients, the general condition and dynamics of complaints were assessed, systolic (SBP) and diastolic blood pressure (DBP) were determined, weight was measured, BMI parameters and waist-to-hip ratio (WC/HR) were calculated. Statistical processing was carried out using the SPSS 13.0 software package to determine the levels of statistical significance. The criterion for statistical significance was p < 0.05. (ND – unreliable).
Special Recommendations
The drug Dibikor can be used in complex therapy of many diseases and pathological conditions. It does not reduce the effects of other medications. But when treating cardiovascular failure with cardiac glycosides, an increased inotropic effect may be observed. This means that you need to reduce their dosage. A similar approach should be applied to drugs that are blockers of “slow” calcium channels.
Dibikor, the instruction manual focuses on this, does not affect psychomotor reactions. Therefore, when carrying out drug therapy, there are no restrictions on driving cars and performing other work operations that require precision and increased concentration.
You can use a broad-spectrum over-the-counter medicine for 3 years from the date of issue. When purchasing dibikor, it is important to pay attention to the date on the cardboard packaging. To store the product, you must choose a place inaccessible to children. Temperature – no higher than 25 °C.
War for Dibikor
Author:
Skrypnik Ksenia
2 minutes
5883
Medical experts have recognized the drug for diabetics “Dibikor”, which is produced by the pharmaceutical company, as ineffective. Efficacy and safety testing has been carried out.
However, despite this, in April 2016, the Moscow Arbitration Court declared the results of studies conducted by medical organizations accredited by the Ministry of Health of the Russian Federation invalid. The court ruled that it must refute the information that Dibikor is ineffective. At the beginning of July, the Ninth Arbitration Court of Appeal made a similar decision.
Twenty years without proof of effectiveness
The main active ingredient of Dibikor is the amino acid taurine, which in many countries is considered a dietary supplement and not a drug. In Russia, however, taurine-based drugs are used in clinical practice. Dibikor is also recognized as a medicine. Pharm registered it back in the 90s, when the requirements for including a drug in the State Register of Medicines were significantly different from those that exist now. At the same time, Dibikor was included in the Register without conducting appropriate clinical trials confirming its effectiveness.
Such trials began only in 2012, and before that, for more than two decades, the drug was prescribed to patients with type 2 diabetes, although international protocols and methods used throughout the world were never used to evaluate its effectiveness. It is assumed that the drug manufacturer agreed to conduct clinical trials due to the fact that after their completion, the drug could be included in the List of Vital and Essential Medicines.
During the audit, all necessary international standards for clinical trials were observed - a double-blind, randomized, placebo-controlled, multicenter clinical trial was conducted. Its results indicate that the effectiveness of the tested drug “Dibikor” (500 mg tablets) is comparable to the effectiveness of placebo. This conclusion was confirmed by specialists from Yaroslavl State Medical University. “From generally accepted clinical positions, this statement from a practical point of view means the ineffectiveness of the drug,” the experts noted. In addition, they emphasized that the study results are reliable and the conclusions are justified.
PIK-PHARMA LLC did not publish data on the ineffectiveness of the drug, but accused ER & DI PHARMA LLC of conducting the study in bad faith and asked the court to declare the results of the clinical trials unreliable.
"Independent expert
Surprisingly, the court twice ruled in favor of the drug manufacturer. Both the Moscow Arbitration Court and the Ninth Arbitration Court turned to an independent expert, A.O Monaenkov from RR MS LLC, whose opinion became decisive, despite the fact that the expert was not an endocrinologist and also had no experience in conducting and analysis of such examinations. This, however, did not prevent him from coming to the conclusion that the defendant committed many significant violations during the conduct of the clinical trials.
Errors were indeed discovered, but they concerned only the accompanying documentation, but not the testing process. Numerous inaccuracies were found in the expert’s report, which were most likely due to his ignorance of terminology and methodology. In addition, journalists managed to find out that the expert was not independent at all - his candidacy was proposed to the court.
The court ruled in favor of the plaintiff and ruled that the results obtained could not be considered reliable. “Therefore, the main conclusion obtained during the clinical trials of the drug Dibikor, that it is not effective and is a “dummy” placebo, is not true.”
However, he is not going to give up and believes that high-quality and conscientious testing of the effectiveness of drugs in compliance with the principles of GCP and in full compliance with Russian legislation and regulations is possible. That is why the company will continue to defend its position in the cassation court.