Pharmacological properties of the drug Metalyse
Pharmacodynamics. Tenecteplase is a recombinant fibrin-specific plasminogen activator derived from natural tissue plasminogen activator (t-Pa) by modifying the protein structure at three sites. Tenecteplase binds to components of the fibrin thrombus and selectively converts thrombus-associated plasminogen into plasmin, which cleaves the fibrin backbone of the thrombus. Compared to native t-PA, tenecteplase has greater fibrin specificity and is more resistant to inactivation under the influence of an endogenous inhibitor (PA-1). After the administration of tenecteplase, dose-dependent absorption of α2-antiplasmin is noted with a further increase in the level of systemic plasmin, which does not affect the effect of plasminogen activation. Reduced concentrations of fibrinogen (less than 15%) and plasminogen (less than 25%) are observed in patients who received tenecteplase at the maximum dose (10,000 units). The formation of antibodies to tenecteplase was not observed (over a period of 30 days). Pharmacokinetics. Tenecteplase is cleared from the circulation by binding to specific receptors in the liver and is metabolized to form peptide fragments. Binding to liver receptors is less compared to native t-PA, which provides a longer half-life. After a single intravenous bolus administration of tenecteplase to patients with acute myocardial infarction, biphasic elimination of the tenecteplase antigen from blood plasma is noted. The clearance of tenecteplase is not dose dependent (within therapeutic doses). The primary half-life is 24±5.5 minutes, which is 5 times higher compared to native t-Pa. The terminal half-life is 129±87 minutes, plasma clearance is 119±49 ml/min. In overweight patients, a moderate increase in the clearance of tenecteplase is observed, and in elderly patients, a slight decrease is observed. In women, drug clearance is lower than in men. The effect of renal and hepatic impairment on the pharmacokinetics of tenecteplase has not been specifically studied.
Description of the drug TENEKTEPLAZA
With caution and after careful assessment of the expected benefits of treatment and
possible risk of bleeding, tenecteplase should be used when systolic blood pressure>160 mmHg. Art.; recent bleeding from the gastrointestinal tract or genitourinary tract (within the last 10 days); recent intramuscular injection (within the last 2 days); in elderly patients (over 75 years old); with low body weight (< 60 kg); cerebrovascular diseases.
If percutaneous coronary intervention (PCI) is planned in accordance with current recommendations (standards of care), full dose tenecteplase should not be pre-administered with a single bolus of up to 4000 IU of unfractionated heparin administered 60-180 minutes before primary PCI in patients with extensive myocardial infarction.
The most common complication associated with the use of tenecteplase is bleeding. Concomitant use of heparin may promote bleeding. After dissolution of fibrin as a result of the use of tenecteplase, bleeding may occur at the sites of recently performed punctures and injections. Therefore, thrombolytic treatment requires careful monitoring of areas where bleeding may occur (including the catheter insertion site, arterial and venous punctures, incisions and injections). The use of rigid catheters, intramuscular injections and unnecessary manipulation should be avoided during treatment with tenecteplase.
If serious bleeding occurs, especially intracranial hemorrhage, concomitant administration of heparin should be discontinued immediately. Protamine sulfate may be prescribed if heparin was prescribed within 4 hours before bleeding occurred. When conservative therapy is ineffective, transfusion medications may be indicated. Transfusion administration of cryoprecipitate, fresh frozen plasma and platelets can be prescribed in accordance with clinical and laboratory parameters, determined repeatedly after each administration. It is advisable to carry out infusion of cryoprecipitate until the fibrinogen concentration reaches about 1 g/l. It is also possible to use antifibrinolytic agents.
Coronary thrombolysis may be accompanied by the occurrence of arrhythmias associated with reperfusion. Reperfusion arrhythmias can lead to cardiac arrest, be life-threatening, and require the use of conventional antiarrhythmic therapy.
Concomitant use of glycoprotein IIb/IIIa antagonists increases the risk of bleeding.
The use of tenecteplase may be accompanied by an increased risk of thromboembolic complications in patients with thrombosis of the left heart, incl. with mitral stenosis or atrial fibrillation.
If an anaphylactoid reaction occurs, the injection should be discontinued.
Use of the drug Metalyse
Prescribed taking into account the patient's body weight, the maximum dose is 10,000 units (50 mg tenecteplase). The volume of the solution corresponding to the effective dose can be determined from the table:
Patient's body weight, kg | Tenecteplase, units | Tenecteplase, mg | Corresponding volume of solution, ml |
| Less than 60 | 6000 | 30 | 6 |
| 60–70 | 7000 | 35 | 7 |
| 70–80 | 8000 | 40 | 8 |
| 80–90 | 9000 | 45 | 9 |
| 90 or more | 10 000 | 50 | 10 |
Administer once intravenously as a bolus (over 5–10 s). To administer Metalyse, you can use an infusion system, which was used only for infusion of 0.9% sodium chloride solution. After injection of Metalyse, the system should be flushed with isotonic solution to ensure the full dose is received. You should not add other medications (even heparin) to the injection solution of the drug or to the IV infusion system. Concomitant therapy Antithrombotic concomitant therapy is recommended in accordance with current international recommendations for the treatment of patients with ST- . Preparation of the solution Metalyse is dissolved by adding the required volume of water for injection from a pre-filled syringe into the vial, which contains the powder for preparing the solution for injection. It is necessary to make sure that the size of the bottle is selected in accordance with the patient’s body weight according to the diagram above, check that the cap on the bottle is intact and only then remove it. Remove the tip from the syringe, immediately screw the syringe filled with solvent into the vial adapter and pierce the vial stopper using the adapter needle. Water should be added to the bottle slowly to avoid foaming and dissolved by shaking gently. The finished solution should be transparent, colorless or pale yellow. Only use a transparent solution free of particles. Immediately before administering the solution, turn the bottle over so that the syringe connected to the bottle is on the bottom. Transfer the appropriate volume of the resulting Metalyse solution into a syringe, taking into account the patient’s body weight. Then separate the syringe from the adapter device. Metalyse should be administered over 5–10 s. You cannot use an intravenous infusion system containing dextrose solution. Any remaining unused solution must be destroyed. Alternatively, the solution can be prepared using a needle, which is included in the package.
Metalyse®
Metalyse® should be prescribed by a specialist with experience in thrombolytic therapy and the ability to monitor its effectiveness. This does not exclude the possibility of using Metalyse® at the prehospital stage. Like other thrombolytic agents, it is recommended that Metalyse® be administered in settings where standard resuscitation equipment and drugs are available.
Primary percutaneous coronary intervention (PCI)
If PCI is planned in accordance with current recommendations (standards of care), Metalyse® at full dose should not be pre-administered with a single bolus of up to 4000 IU of unfractionated heparin administered 60-180 minutes before primary PCI in patients with large infarction myocardium.
Bleeding
The most common complication associated with the use of Metalyse® is bleeding. Concomitant use of heparin may promote bleeding. After dissolution of fibrin as a result of the use of Metalyse®, bleeding may occur at the sites of recently performed punctures and injections. Therefore, thrombolytic treatment requires careful monitoring of areas where bleeding may occur (including the catheter insertion site, arterial and venous punctures, incisions and injections). The use of rigid catheters, intramuscular injections and unnecessary manipulations should be avoided during treatment with Metalyse®.
If serious bleeding occurs, especially intracranial hemorrhage, concomitant administration of heparin should be discontinued immediately. Protamine sulfate may be prescribed if heparin was prescribed within 4 hours before bleeding occurred. When conservative therapy is ineffective, transfusion medications may be indicated. Transfusion administration of cryoprecipitate, fresh frozen plasma and platelets can be prescribed in accordance with clinical and laboratory parameters, determined repeatedly after each administration. It is advisable to carry out infusion of cryoprecipitate until the fibrinogen concentration reaches about 1 g/l. It is also possible to use antifibrinolytic agents.
Arrhythmias
Coronary thrombolysis may be accompanied by the occurrence of arrhythmias associated with reperfusion. Reperfusion arrhythmias can lead to cardiac arrest, be life-threatening, and require the use of conventional antiarrhythmic therapy.
Glycoprotein IIb/IIIa antagonists
Concomitant use of glycoprotein IIb/IIIa antagonists increases the risk of bleeding.
Thromboembolism
The use of Metalyse® may be accompanied by an increased risk of thromboembolic complications in patients with thrombosis of the left heart, incl. with mitral stenosis or atrial fibrillation.
Hypersensitivity
The formation of antibodies to the tenecteplase molecule after treatment was not detected. However, there is no experience with repeated use of Metalyse®. Anaphylactoid reactions associated with the use of Metalyse® have been observed rarely and could be the cause of hypersensitivity to tenecteplase, gentamicin (trace amounts, used in the manufacturing process) or to any other excipient. If an anaphylactoid reaction occurs, the injection should be discontinued.
Prepared solution
The prepared solution remains stable for 24 hours at a temperature of 2-8°C and for 8 hours at a temperature of 30°C. From a microbiological point of view, the solution should be used immediately after preparation. If the solution was prepared in advance and not administered, the period and conditions of its storage before use become the responsibility of the specialist prescribing the drug; The shelf life usually does not exceed 24 hours at a temperature of 2-8°C and 8 hours at a temperature of 30°C. Partially used solution must be destroyed.
Contraindications to the use of the drug Metalyse
Thrombolytic therapy is associated with a risk of bleeding. Metalyse is contraindicated in the following cases:
- hypersensitivity to the active substance or any other ingredient of the drug;
- significant bleeding currently or over the past 6 months, a history of hemorrhagic diathesis;
- in case of receiving concomitant oral anticoagulant therapy (INR 1.3);
- the presence of any disorders of the central nervous system (for example, tumor, aneurysm, intracranial or spinal surgery);
- severe hypertension (arterial hypertension), uncontrollable;
- major surgery, solid organ biopsy, significant trauma within the past 2 months (including any trauma preceding a true myocardial infarction), recent head or skull trauma;
- prolonged or traumatic cardiopulmonary resuscitation (2 min) over the past 2 weeks;
- severe liver dysfunction, including liver failure, cirrhosis, portal hypertension (esophageal varices) and active hepatitis;
- peptic ulcer;
- arterial aneurysm and known arterial/venous malformation;
- tumor with an increased risk of bleeding;
- acute pericarditis and/or subacute bacterial endocarditis;
- acute pancreatitis;
- hemorrhagic stroke or stroke of unknown origin during any period;
- ischemic stroke or transient ischemic attack within the last 6 months.
Side effects of the drug Metalyse
As with other thrombolytic agents, bleeding is the most common side effect associated with the use of Metalyse. Bleeding from any location or body cavity may occur, which may result in permanent disability or death. The types of bleeding associated with thrombolytic therapy can be divided into two main categories:
- superficial bleeding, usually from the injection site;
- internal bleeding of any location or body cavity.
Neurological symptoms such as drowsiness, aphasia, hemiparesis, and seizures may be associated with intracranial hemorrhage. The reported incidence is based on relevant clinical trial data involving 8258 patients who received Metalyse therapy for myocardial infarction. Therapy with Metalyse in some cases can lead to the development of embolism with cholesterol fragments or thromboembolism. These systematizations of cases of embolization with cholesterol crystals are based on the fact that in the largest clinical trial involving more than 14 thousand patients, not a single such case was identified. Immune system disorders (1/1000, ≤1/100): anaphylactoid reactions (including skin rash, urticaria, bronchospasm, laryngeal edema). CNS disorders (1/1000, ≤1/100): intracranial hemorrhage (for example, cerebral hemorrhage, intracranial hematoma, hemorrhagic stroke, hemorrhagic transformation of stroke, subarachnoid hemorrhage). Visual impairment (≤1/10,000): intraocular hemorrhage. Cardiac disorders (1/10): reperfusion arrhythmias (asystole, accelerated idioventricular arrhythmia, arrhythmia, extrasystole, atrial fibrillation, atrioventricular block (from stage I to complete), bradycardia, tachycardia, ventricular extrasystole, ventricular fibrillation, ventricular tachycardia) are noted in direct temporal relationship with the use of Metalise. Reperfusion arrhythmias can lead to life-threatening cessation of cardiac activity and may therefore require the use of traditional antiarrhythmic therapy; (1/10,000, ≤1/1000): hemopericardium. Vascular disorders (1/10): bleeding; (1/1000, ≤1/100): embolism (thrombotic embolism). Respiratory, thoracic and mediastinal disorders (1/100, ≤1/10): epistaxis; (1/1,000, ≤1/100): pulmonary hemorrhage. Gastrointestinal disorders (1/100, ≤1/10): gastrointestinal bleeding (eg gastric, ulcerative, rectal bleeding, hematemesis, melena, oral bleeding), nausea, vomiting; (1/1000, ≤1/100): bleeding in the retroperitoneal space (eg retroperitoneal hematoma). Skin and subcutaneous tissue disorders (1/100, ≤ 1/10): ecchymosis. Urinary system disorders (1/100, ≤1/10): urogenital bleeding (eg hematuria, bleeding from the genitourinary organs). General disorders and reactions at the injection site (1/10): superficial bleeding, usually from a puncture or damaged vessel. Clinical examination (1/10): decreased blood pressure; (1/100, ≤1/10): increased body temperature. Disturbances, poisonings and procedural complications (≤1/10,000): fat embolism (cholesterol crystal embolization), which can lead to corresponding consequences in the associated organs. Surgical and medical procedures: (1/100, ≤1/10): need for whole blood transfusion.
Description of the drug METALIZE
With caution and after careful assessment of the expected benefits of treatment and
possible risk of bleeding, tenecteplase should be used when systolic blood pressure>160 mmHg. Art.; recent bleeding from the gastrointestinal tract or genitourinary tract (within the last 10 days); recent intramuscular injection (within the last 2 days); in elderly patients (over 75 years old); with low body weight (< 60 kg); cerebrovascular diseases.
If percutaneous coronary intervention (PCI) is planned in accordance with current recommendations (standards of care), full dose tenecteplase should not be pre-administered with a single bolus of up to 4000 IU of unfractionated heparin administered 60-180 minutes before primary PCI in patients with extensive myocardial infarction.
The most common complication associated with the use of tenecteplase is bleeding. Concomitant use of heparin may promote bleeding. After dissolution of fibrin as a result of the use of tenecteplase, bleeding may occur at the sites of recently performed punctures and injections. Therefore, thrombolytic treatment requires careful monitoring of areas where bleeding may occur (including the catheter insertion site, arterial and venous punctures, incisions and injections). The use of rigid catheters, intramuscular injections and unnecessary manipulation should be avoided during treatment with tenecteplase.
If serious bleeding occurs, especially intracranial hemorrhage, concomitant administration of heparin should be discontinued immediately. Protamine sulfate may be prescribed if heparin was prescribed within 4 hours before bleeding occurred. When conservative therapy is ineffective, transfusion medications may be indicated. Transfusion administration of cryoprecipitate, fresh frozen plasma and platelets can be prescribed in accordance with clinical and laboratory parameters, determined repeatedly after each administration. It is advisable to carry out infusion of cryoprecipitate until the fibrinogen concentration reaches about 1 g/l. It is also possible to use antifibrinolytic agents.
Coronary thrombolysis may be accompanied by the occurrence of arrhythmias associated with reperfusion. Reperfusion arrhythmias can lead to cardiac arrest, be life-threatening, and require the use of conventional antiarrhythmic therapy.
Concomitant use of glycoprotein IIb/IIIa antagonists increases the risk of bleeding.
The use of tenecteplase may be accompanied by an increased risk of thromboembolic complications in patients with thrombosis of the left heart, incl. with mitral stenosis or atrial fibrillation.
If an anaphylactoid reaction occurs, the injection should be discontinued.
Special instructions for the use of the drug Metalyse
Treatment should begin as soon as possible after the onset of symptoms. Metalyse should be prescribed by a physician with experience in the use of thrombolytic therapy and the ability to monitor its use. This does not exclude the possibility of using Metalyse at the prehospital stage. When prescribing Metalyse, it is recommended in all cases to have standard equipment and medications for resuscitation. Bleeding The most common complication with Metalyse therapy is bleeding. Concomitant use of heparin may promote bleeding. Since fibrin dissolves during Metalyse therapy, bleeding may occur at the site of a recent vascular puncture. Therefore, when carrying out thrombolytic therapy, possible sites of bleeding should be monitored (sites of insertion of catheters, arterial and venous puncture, venesection and puncture biopsy). During Metalyse therapy, you should avoid installing rigid catheters, performing intramuscular injections and traumatic manipulations that are not vital. If serious bleeding occurs, especially intracerebral, simultaneous use of heparin should be stopped immediately, and protamine should be prescribed if heparin was administered within 4 hours before the onset of bleeding. Some patients in whom conservative measures are ineffective may be given a blood transfusion. The advisability of transfusion of cryoprecipitate, fresh frozen plasma and platelets should be considered taking into account clinical and laboratory parameters. The expected target fibrinogen level after cryoprecipitate infusion is 1 g/L. The need for antifibrinolytic agents should also be considered. The advisability of prescribing Metalyse therapy should be carefully assessed by comparing the potential risk of bleeding and the expected benefit in the presence of the following conditions: systolic blood pressure 160 mm Hg. Art.; recent bleeding in the gastrointestinal tract or genitourinary organs (within the last 10 days); any recent (within the last 2 days) intramuscular injection; old age (over 75 years); low body weight (≤60 kg); diseases of cerebral vessels. Arrhythmia Coronary thrombolysis can cause the development of reperfusion arrhythmia. Glycoprotein IIb/IIIa antagonists Concomitant use of glycoprotein IIb/IIIa antagonists increases the risk of bleeding. Thromboembolism The use of Metalyse may increase the risk of thromboembolism in patients with a blood clot on the left side of the heart, such as mitral stenosis or atrial fibrillation. Repeated administration The formation of antibodies to tenecteplase after treatment was not observed. There is no experience with re-prescribing Metalyse. During pregnancy and breastfeeding. There is no experience with the use of Metalyse during pregnancy. The ratio of the expected therapeutic effect for the mother (in the event of myocardial infarction during pregnancy) with the potential risk to the fetus should be assessed. It is not known whether tenecteplase passes into breast milk. The ability to influence reaction speed when driving vehicles or other mechanisms has not been studied.
Metalize
The drug should be administered by a physician with experience in thrombolytic therapy and the ability to monitor its effectiveness. This does not exclude the possibility of using tenecteplase at the prehospital stage. It is recommended to administer the drug in conditions where standard resuscitation equipment and drugs are available.
Bleeding is the most common complication when using the drug. Concomitant use of heparin may promote bleeding. After dissolution of fibrin as a result of the use of tenecteplase, bleeding may occur at the sites of recently performed punctures and injections. Therefore, thrombolytic treatment requires careful monitoring of areas where bleeding may occur (including the catheter insertion site, arterial and venous punctures, incisions and injections). The use of rigid catheters, intramuscular injections and unnecessary manipulations during treatment should be avoided.
If serious bleeding occurs, especially intracranial hemorrhage, concomitant administration of heparin should be discontinued immediately. The possibility of prescribing protamine should be remembered if heparin was prescribed within 4 hours before bleeding occurred. In rare cases when the listed conservative treatment measures are ineffective, rational administration of transfusion drugs may be indicated. Transfusion administration of cryoprecipitate, fresh frozen plasma and platelets can be prescribed in accordance with clinical and laboratory parameters, determined repeatedly after each administration. It is advisable to carry out infusion of cryoprecipitate until the fibrinogen concentration reaches about 1 g/l. It is also possible to use antifibrinolytic agents.
Coronary thrombolysis may be accompanied by the occurrence of arrhythmias associated with reperfusion.
There is no experience with the use of glycoprotein llb/llla antagonists during the first 24 hours after initiation of treatment.
The use of tenecteplase may be accompanied by an increased risk of thromboembolic complications in patients with thrombosis of the left heart, incl. with mitral stenosis or atrial fibrillation.
The formation of antibodies to the tenecteplase molecule after treatment was not detected, but there is no experience with repeated use of tenecteplase.
There is no experience with the use of the drug in pregnant women. There is no data on the excretion of tenecteplase in breast milk. The degree of possible risk and expected benefit should be weighed when prescribing the drug in the event of acute myocardial infarction during pregnancy and lactation.
The physical and chemical properties of the prepared solution are stable for 24 hours at a temperature of 2-8 degrees C and for 8 hours at a temperature of 30 degrees C. From a microbiological point of view, the solution should be used immediately after preparation. If the solution was not used immediately, the period and conditions of its storage before use become the responsibility of the doctor prescribing the drug.
Drug interactions Metalyse
Specific studies of the interaction of Metalyse with other medications that are commonly used to treat patients with acute myocardial infarction have not been conducted. However, an analysis of data from more than 12 thousand patients who were treated during phases 1, 2 and 3 did not reveal any clinically significant interactions between co-administered drugs in patients with acute myocardial infarction and Metalyse. Anticoagulants and antiplatelet agents may increase the risk of bleeding before, during and after Metalyse therapy. Incompatibility. Metalyse is incompatible with dextrose solution. Do not add other drugs to Metalyse solution.
Benefits of tenecteplase in the treatment of ST-segment elevation myocardial infarction
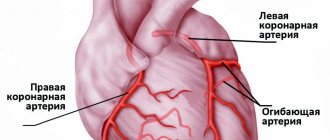
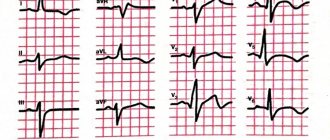
There is no doubt that performing PCI within the first 12 hours from the onset of symptoms is the “gold standard” for the treatment of myocardial infarction (MI), provided that the procedure is performed within the first hour of initial contact with a medical professional. However, in our country, with its vast territory, low population density in many areas, an insufficiently developed transport network and, unfortunately, the absence of a 24-hour catheterization service in many hospitals, compliance with the conditions for providing this type of medical care is limited. Thus, according to various sources, in 2011–2012 in the Russian Federation, the percentage of primary PCI performed in patients with NSTE-ACS was 5%. An alternative reperfusion strategy is TLT. Large foreign studies, including the CAPTIM study, have shown that administration of a thrombolytic within the next 2 hours from the onset of NSTE-ACS is more effective than PCI, improving the immediate and long-term prognoses of patients with MI [1, 2]. At the same time, it should be noted that at the prehospital stage, TLT should be carried out by specially equipped emergency medical teams (Class of recommendations IIA, level of evidence A) [3].
Currently, one of the methods for quickly restoring blood flow is prehospital thrombolysis followed by PCI, which is called the “pharmacoinvasive approach.” The effectiveness of such tactics was studied in the international multicenter prospective open study STREAM (Strategic Reperfusion Early after Myocardial Infarction), conducted from March 2008 to July 2012 [4, 5]. The study included 1892 patients with a symptom duration of up to 3 hours and recorded ECG signs of NSTE-ACS (ST segment elevation ≥2 mm in at least two consecutive standard or precordial leads), who could not undergo primary PCI within 1 hour after the first contact with medical staff. According to the results of randomization performed at the prehospital stage, the first group of patients (TLT group) was administered tenecteplase in combination with antiplatelet agents (clopidogrel in a saturating dose of 300 mg and acetylsalicylic acid 150–325 mg) and anticoagulants (enoxaparin - 30 mg intravenously as a bolus, followed by subcutaneous administration at a dose of 1 mg per 1 kg of body weight; in patients 75 years of age or older - 0.75 mg per 1 kg of body weight every 12 hours). The group of patients with clinically and documented ineffective TLT underwent emergency coronary angiography and “rescue” PCI on the infarct-related artery. If thrombolysis was effective, angiography was performed within 6 to 24 hours after administration of the thrombolytic. The median time between the onset of clinical manifestations of MI and first medical contact and randomization was similar in both groups. The median time between the first symptoms of ACS and the start of reperfusion therapy in the TLT group (bolus administration of tenecteplase) and in the primary PCI group was 100 and 178 minutes, respectively (p<0.001). The median time to coronary angiography (CAG) was expectedly longer in the TLT group compared to the PCI group and amounted to 2.2 hours in 36% of patients in the TLT group in the “rescue” PCI subgroup and 17 hours for the remaining 64% of patients in this group. Patients in both groups did not differ statistically in basic characteristics. There were no statistically significant relationships between certain characteristics of patients and the treatment tactics used. The duration of observation was 30 days.
The primary endpoint of the study included a composite of all-cause mortality and the incidence of cardiogenic shock, congestive heart failure, or recurrent myocardial infarction. At 30 days of follow-up, there was no statistically significant difference in the incidence of the primary endpoint between the tenecteplase and primary PCI groups (12.4% vs. 14.3%, respectively, p=0.21). Moreover, a decrease in the frequency of the primary end point in the pharmacoinvasive strategy group was noted mainly due to a decrease in the risk of developing cardiogenic shock and congestive heart failure. The overall clinical outcome during the subsequent year of follow-up reflected low 30-day mortality in both the TLT (4.6%) and primary PCI (4.4%) groups; overall mortality in the presented groups also did not have statistically significant differences, although was slightly higher in the pharmacoinvasive approach group (6.7% vs. 5.9%). It was also found that in the group of effective TLT, restoration of the patency of the coronary arteries was significantly more often detected (with CAG, the blood flow in the infarct-related coronary artery in 13.2% of patients corresponded to the 2nd degree and in 72.8% of patients - to the 3rd degree according to TIMI classification), in the group of patients with ineffective TLT, blood flow in the infarct-related coronary artery in 46.5% of cases corresponded to grade 0 or 1 according to the TIMI classification. The rate of restoration of blood flow after primary PCI or in the pharmacoinvasive approach group was almost the same. The greatest advantages of the pharmacoinvasive approach were noted in patients with lower MI.
In addition, the study analyzed the incidence of ischemic stroke, intra- and extracranial bleeding and other adverse outcomes, the incidence of which was similar in both groups. At the early stages of the study, attention was drawn to a tendency toward an increase in the incidence of intracranial bleeding in the TLT group in patients over 75 years of age, which required a dose adjustment of tenecteplase. After halving the drug dose in patients over 75 years of age, the stroke rate was comparable to that in the PCI group (0.5% vs. 0.3%, p=0.45). Similar results were obtained in previously published studies on the use of TLT in the first hours of STEMI followed by PCI [6, 7]. The effectiveness and safety of the pharmacoinvasive treatment strategy was also proven in the TRANSFER-AMI study (Trial of Routine Angioplasty and Stenting after Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction), completed in 2009. During the study, 1059 patients with NSTE-ACS underwent thrombolysis in a treatment center , not equipped with an endovascular service, with further division of patients into two groups: a group of standard therapy (“salvage” or delayed PCI) and a group of pharmacoinvasive strategy (using tenecteplase), the patients of which were immediately transported to the PCI center. The average time from thrombolytic administration to PCI in the pharmacoinvasive group was 3.9 hours. A significant reduction in mortality, recurrent myocardial infarction, early post-infarction angina, acute left ventricular failure and cardiogenic shock was demonstrated within 30 days in the pharmacoinvasive approach group (relative risk for early PCI was 0.64 (95% CI 0.47 to 0.87; p=0.004)). However, in this group of patients there was also an increase in the proportion of moderate bleeding in accordance with GUSTO criteria compared to the standard therapy group (13.0% versus 9%, p = 0.04). At the same time, no significant differences were found in the incidence of major and minor bleeding according to the TIMI criteria [8, 9].
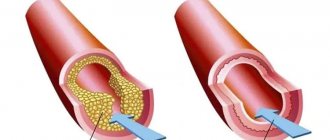
In addition, a report published in 2013 by Larsen et al. includes results from the Minneapolis registry of 2634 patients with hyperacute STEMI using half-dose fibrinolytic in combination with a loading dose of clopidogrel (600 mg) and unfractionated heparin (UFH) followed by immediate PCI, provided there was no facility within 60 miles . Analysis of primary endpoints in the form of mortality, number of strokes, major bleeding, recurrent myocardial infarction demonstrated similar results in the primary PCI and pharmacoinvasive strategy groups, despite a significantly longer door-to-balloon time in the second group. The need for additional mechanical reperfusion in the group of patients with effective TLT, according to the study, was 80–85% [10]. To summarize, the studies conducted, including the results of the STREAM study, have proven the effectiveness of a combined pharmacoinvasive strategy that allows the benefits of TLT to be used along with high-quality restoration of coronary blood flow during subsequent PCI. Moreover, the above works have proven the indisputable feasibility of using thrombolytics at the prehospital stage, which ensures rapid restoration of coronary blood flow. The findings made it possible to concentrate on choosing the optimal drug, the advantages of which should be effectiveness, safety and ease of use. Achievements of genetic engineering in the form of DNA modification of three amino acid sequences led to the creation of a recombinant plasminogen activator - the drug tenecteplase, which has the most pronounced thrombolytic activity (increasing the half-life in blood plasma from 4 to 20 minutes), fibrin specificity and resistance to the influence of the endogenous plasminogen activator inhibitor - 1(PAI-1). The molecular weight of tenecteplase is 65,000 kDa.
The inhibitory capacity of PAI-1 with tenecteplase is 80 times less than other molecules used in clinical practice. An increase in fibrinolytic activity occurs due to the binding of the fibrin component of the thrombus and the selective activation of the plasminogen-plasmin system, which, in turn, leads to the destruction of the fibrin base of the thrombus. Limiting the activity of plasmin in the fibrin substrate avoids the breakdown of fibrinogen, coagulation factors V and VIII, as well as α2-antiplasmin (a plasmin inhibitor in the liquid phase) and thereby reduces the risk of major bleeding. These characteristics are great advantages for using the drug in the treatment of STEMI. It should be noted that a large number of studies have been devoted to studying the properties of the drug, including large multicenter studies over the past 15 years. One of the advantages of tenecteplase is its ease of use. It is currently the only thrombolytic drug given as a single bolus injection. Thus, the results of the studies TIMI 10A, TIMI 10B, ASSENT-1 showed an equivalent dose-dependent effect of a single intravenous bolus administration of tenecteplase (dose calculation depending on body weight) and accelerated administration of alteplase in the treatment of STEMI in relation to the restoration of blood flow in the coronary arteries [11, 12].
The data obtained were confirmed in a large multicenter, randomized, double-blind study, ASSENT-2 (Assessment of the Safety of a New Thrombolytic), which included 16,949 patients with STEMI in the first 6 hours from the onset of symptoms [13]. Patients were divided into 2 groups, receiving either an IV bolus of tenecteplase depending on body weight (less than 60 kg - 30 mg; 60-69.9 kg - 35 mg; 70-79.9 kg - 40 mg; 80-89 .9 kg - 45 mg; more than 90 kg - 50 mg) for 5-7 minutes, or accelerated administration of alteplase in combination with intravenous infusion of UFH. The primary end point was mortality within the next 30 days, which was virtually the same in both groups (6.18% versus 6.15%). These groups also did not differ in the incidence of serious complications, including the incidence of hemorrhagic stroke (0.93% in the tenecteplase group, 0.94% in the alteplase group). At the same time, in the tenecteplase group there was a significantly lower number of major non-intracranial bleedings (4.66 versus 5.94%; p = 0.0002), including bleeding with subsequent blood transfusion (4.25 versus 5.49% ; p=0.0002), and bleeding of any severity (26.43 vs. 28.95%; p=0.0003). In addition, a reduced risk of acute heart failure was found with tenecteplase compared with alteplase (Killip grade >16.1% vs. 7.0%, respectively, p=0.025).
Thus, the study proved the effectiveness and safety of tenecteplase therapy in the form of a single bolus administration compared with a complex alteplase infusion regimen, regardless of age, gender, location of the infarction, class of heart failure and the presence of diabetes mellitus, which further improved the prognosis of the disease.
Conclusion The results of the STREAM study once again emphasized that prehospital thrombolysis in combination with modern antithrombotic therapy and subsequent timely coronary angiography and PCI (pharmacoinvasive strategy) makes it possible to achieve effective restoration of myocardial perfusion in patients with acute STEMI in the early stages of the onset of the disease and reduce the percentage of recurrent infarctions in patients with effective TLT after elimination of residual stenosis. The data obtained enable a balanced approach to choosing a treatment strategy for STEMI, including optimal drug therapy. Today, in our opinion, the most promising thrombolytic can be identified - tenecteplase, which is the most convenient to use, is not inferior in its effectiveness to tissue plasminogen activator (alteplase), and is completely superior in safety.
References 1. Steg et al. Impact of Time to Treatment on Mortality After Prehospital Fibrinolysis or Primary Angioplasty: Data From the CAPTIM Randomized Clinical Trial // Circulation. 2003. Vol. 108. P. 2851–2856. 2. International Experts Workshop on Early Treatment Strategies for Acute Myocardial Infarction. Budapest, July 4–6 2008. https://www.metalyse.com/img/ download/ mi_news_budapest08.pdf 3. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. A Report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines // JACC. 2013. Vol. 61. P. 485–510. 4. Armstrong PW, Gershlick AH, Goldstein P. et al. For the STREAM Investigative Team. Fibrinolysis or Primary PCI in ST-Segment Elevation Myocardial Infarction. // N. Engl. J. Med. 2013. DOI: 10.1056/NEJMoa1301092. 5. Armstrong PW, Gershlick A, Goldstein P et al. On behalf of the STREAM Steering Committee. The Strategic Reperfusion Early After Myocardial Infarction (STREAM) study // Am. Heart J. 2010. Vol. 160. P. 30–35. 6. Staroverov I.I. Myocardial infarction: new prospects for fibrinolytic therapy // Doctor.Ru. 2004. No. 4, reprint. 7. Bonnefoy E., Lapostolle F., Leizorovicz A. et al. Primary angioplasty versus prehospital fibrinolysis in acute myocardial infarction: a randomized study // Lancet. 2002. Vol. 360. P. 825–829. 8. Warren J. et al. Routine Early Angioplasty after Fibrinolysis for Acute Myocardial Infarction for the TRANSFER-AMI Trial Investigators // N. Engl. J. Med. 2009. Vol. 360. P. 2705–2718, June 25, 2009 DOI: 10.1056/NEJMoa0808276. 9. GUSTO IIB investigators. A comparison of reteplase with alteplase for acute myocardial infarction // N. Engl. J. Med. 1997. Vol. 337. P.1118–1123. 10. Larson DM, Duval S, Sharkey SW et al. Safety and efficacy of a pharmaco-invasive reperfusion strategy in rural ST-elevation myocardial infarction patients with expected delays due to long distance transfers // Eur. Heart J. doi:10.1093/eurheartj/ehr403. Publish ed online ahead of print 31 October 2011. 11. Antman EM, Hand M., Armstrong PW et al. 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: Developed in Collaboration With the Canadian Cardiovascular Society Endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004, Writing Committee // Circulation. 2008. Vol. 117. P. 296–329. 12. Van de Werf F., Cannon CP, Luyten A. et al. Safety assessment of single-bolus administration of TNK tissue-plasminogen activator in acute myocardial infarction: the ASSENT- 1 trial. The ASSENT-1 Investigators // Am Heart J. 1999. Vol.137. P. 786–791. 13. Assessment of the Safety and Efficacy of a New Thrombolytic Investigators. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomized trial // Lancet. 1999. Vol. 354. P. 716–722.