Streptococcus pneumoniae (pneumococcus)
Pneumococcus
(lat.
Streptococcus pneumoniae
) - a type of gram-positive facultative anaerobic α-hemolytic bacteria.
The cells of Streptococcus pneumoniae
, like other streptococci, have a spherical shape.
Most often they are located in pairs, but in liquid media they form chains. Streptococcus pneumoniae
is the most common (20–60%) causative agent of a dangerous disease - community-acquired pneumonia, the mortality rate of which is 5%.
In addition, Streptococcus pneumoniae
can cause human diseases such as otitis media, acute non-purulent sinusitis, rhinitis, laryngitis, bronchitis, meningitis, sepsis, osteomylitis, septic arthritis, endocarditis, peritonitis and others.
Streptococcus pneumoniae
is the second (after
Haemophilus influenzae
) in terms of prevalence and importance of pneumotropic microorganisms in the microbial spectrum for chronic bronchopulmonary diseases in children during the period of exacerbation.
Pneumonia
Pneumonia is a form of acute respiratory infection that affects the lungs.
In pneumonia, the alveoli of the lungs fill with pus and fluid, making breathing painful and limiting oxygen supply. Pneumonia is the leading single infectious cause of death in children worldwide. In 2013, 935 thousand children under the age of 5 died from pneumonia. It accounts for 15% of all deaths in children under 5 years of age worldwide.
There are several ways pneumonia can spread. Bacteria that are usually present in a child's nose or throat can infect the lungs when inhaled. They can also be spread through respiratory droplets from coughing or sneezing. In addition, pneumonia can be transmitted through blood, especially during or immediately after childbirth.
In children under 5 years of age with symptoms of cough and/or difficulty breathing, with or without fever, the diagnosis of pneumonia is made if there is rapid breathing or lower chest indrawing, if the chest retracts or recedes when inhaling (in a healthy person When you inhale, the chest expands.
Pneumonia caused by bacteria can be treated with antibiotics. The antibiotic of choice is amoxicillin dispersible tablets (WHO Inf. Bulletin No. 131).
Worsening of the epidemiological situation in Moscow regarding community-acquired pneumonia of pneumococcal etiology in 2016.
The Resolution of the Chief State Sanitary Doctor for the city of Moscow dated July 29, 2016 No. 8 states:
- for 6 months of 2016, compared to the same period, there was an increase in the incidence of community-acquired pneumonia by 27%, including pneumococcal etiology - by 1.9 times
- Mortality rates among Muscovites from community-acquired pneumonia increased 1.5 times, mortality rates increased 2 times
- the largest number of cases are among Muscovites over 40 years of age suffering from chronic diseases
- The proportion of hospitalized patients with community-acquired pneumonia is growing:
- in 2014 - 70.9%
- in 2015 - 75.8%
- for 6 months of 2016 - 77.0%
- 20-25% have chronic obstructive pulmonary disease
- up to 15.8% have coronary heart disease
- 14.3% - chronic cardiovascular failure
- 9.6% - diabetes mellitus
Streptococcus pneumoniae in the taxonomy of bacteria
According to modern concepts, the species Streptococcus pneumoniae
belongs to the genus of streptococci, (
Streptococcus
), which is included in the family
Streptococcaceae
, order
Lactobacillales
, class
Bacilli
, phylum
Firmicutes
, <group without rank>
Terrabacteria group
, kingdom Bacteria.
Antibiotics active against Streptococcus pneumoniae. Vaccines
The following antimicrobial agents (from those presented in this reference book) are active against Streptococcus pneumoniae
: azithromycin, clarithromycin, levofloxacin, oflaxacin, josamycin, doxycycline, vancomycin, amoxicillin, moxifloxacin, roxithromycin.
To ciprofloxacin Streptococcus pneumoniae
moderately sensitive.
To prevent pneumococcal infections, polysaccharide conjugate adsorbed vaccines “Prevenar” and “Prevenar 13” from Pfizer HCP Corporation (USA) are used. In Russia, the production of the 13-valent vaccine Prevenar 13 has been localized. In 2016, the pneumococcal polysaccharide vaccine Pneumovax 23 produced by Merck & Co. was registered in Russia. for active immunization against pneumococcal infection in adults and children over 2 years of age, patients with chronic diseases or reduced immunity, containing a mixture of highly purified capsular polysaccharides from the 23 most common and invasive serotypes of Streptococcus pneumoniae:
1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V,10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, 33F.
Streptococcus pneumoniae in ICD-10
Streptococcus pneumoniae
is mentioned in "Class I. Certain infectious and parasitic diseases (A00-B99)" of the International Classification of Diseases ICD-10, in the blocks:
- “A30-A49 Other bacterial diseases”, under the heading “A40.3 Septicemia caused by Streptococcus pneumoniae
. Pneumococcal septicemia" - “B95-B98 Bacterial, viral and other infectious agents” and has the heading code “B95.3 Streptococcus pneumoniae
as a cause of diseases classified in other headings”. This code is intended to be used as an additional code when it is appropriate to identify infectious agents of diseases classified in other headings.
and also in "Class X. Diseases of the respiratory system (J00-J99)":
- in block “J10-J18 Influenza and pneumonia”, “J13 Pneumonia caused by Streptococcus pneumoniae
.
Bronchopneumonia caused by S. pneumoniae
»
On the website GastroScan.ru in the “Literature” section there are subsections “Microflora, microbiocenosis, dysbiosis (dysbacteriosis)” and “Bronchopulmonary diseases associated with gastrointestinal diseases”, containing publications for healthcare professionals.
In the video “Semiotics of cough in children,” aimed at pediatricians, Professor D.Yu. Ovsyannikov addresses problems associated with bacterial bronchitis in children, associated, in particular, with S. pneumoniae
.
Back to section
Mechanisms of S. pneumoniae resistance and antibiotic strategies
Streptococcus pneumoniae is the main causative agent of community-acquired pneumonia, meningitis, sepsis, bacteremia and otitis media. Penicillin, which entered clinical practice in the early 1940s, showed extremely high clinical efficacy for pneumococcal infections, but already in 1967 the first confirmed reports of the isolation of isolates resistant to it appeared. Another 10 years later, an outbreak of pneumococcal infection in South Africa confirmed the existence of multiple resistance of this pathogen to penicillin, tetracycline, erythromycin, chloramphenicol, clindamycin and rifampicin. Studies have shown that antibiotic-resistant isolates of S. pneumoniae are also isolated from patients who have not previously received antibiotic therapy. The spread of antibiotic resistance, associated with excessive and sometimes unjustified use of antibiotics, has become a serious medical problem.
American pharmacologists and infectious disease specialists published a review devoted to the mechanisms of pneumococcal resistance to antibacterial drugs of different pharmacological groups. These mechanisms determine how quickly the presence of genetic markers of antibiotic resistance in bacteria becomes clinically significant, how easily they spread in the population, and whether we can overcome resistance by changing the regimen of using the same antibiotics. Ultimately, the choice of first-line drug for empirical therapy is determined not only by the current level of susceptibility of S. pneumoniae, but also by the impact of such choice on the further evolution of antibiotic resistance.
Beta-lactam drugs are most often used to treat pneumococcal infections. The definition of susceptibility of S. pneumoniae, which is regularly revised by the US Clinical and Laboratory Standards Institute (CLSI), has undergone significant changes over time. Thus, in 2002, separate criteria for the sensitivity of isolates isolated from pneumococcal meningitis and “non-meningeal” infections to parenteral cephalosporins of the 3rd and 4th generations were introduced. In 2008, more flexible criteria for sensitivity to penicillin were established for “non-meningeal” isolates of S. pneumoniae, as reports accumulated on the high effectiveness of penicillin in pneumonia caused by pneumococci, which were considered insensitive according to the previous criteria. Additionally, CLSI defined susceptibility criteria for different routes of penicillin administration: parenteral and oral. These changes were due to the fact that absorption of the antibiotic and its penetration into the site of infection largely determine the clinical effect. New approaches to assessing susceptibility have led to a decrease in the prevalence of penicillin-resistant S. pneumoniae strains. It should also be noted that European countries in recent years have been switching to EUCAST (European Committee for Antimicrobial Susceptibility Testing) susceptibility criteria, which are somewhat different from the CLSI criteria. Therefore, when comparing data on penicillin resistance of pneumococci in different years and in different countries, it is necessary to take into account which criteria were used by the authors of each publication.
Resistance of S. pneumoniae to penicillin and other beta-lactams develops due to a mutation leading to structural changes in the penicillin binding protein (PBP). The PBP family includes enzymes responsible for the synthesis of peptidoglycans in bacterial cells. When they are blocked by penicillin, the integrity of the cell wall is disrupted, which leads to cell lysis and death. Six types of PSP have been described in S. pneumoniae; three of them are of greatest importance for the development of resistance: PSB1a, PSB2x and PSB2b, while the mutation of the PSB1a gene only leads to a decrease in sensitivity to penicillin.
The sensitivity of bacteria to antibiotics in vitro is assessed by the value of the minimum inhibitory concentration (MIC) - this is the concentration of the drug that leads to visible suppression of the growth of the pathogen in the test medium. Since pneumococcus has several types of PBP, a single mutation is not enough to form a high level of resistance to penicillin. The accumulation of mutations leads to a gradual and stepwise increase in MIC, and a combination of several mutations is necessary for the development of clinically significant resistance to penicillins. This mechanism can largely be overcome by increasing the dose of the drug or changing the regimen of its use. The reduced sensitivity or resistance of S. pneumoniae to penicillin extends to other beta-lactam antibiotics. To a lesser extent, the binding of parenteral cephalosporins of the 4th and 5th generations and carbapenems is impaired.
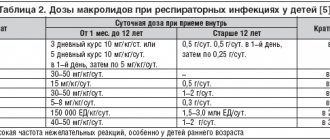
The group of macrolides is united by the presence in the structure of the molecule of a lactone ring, which can consist of 12–16 atoms. The mechanism of action of these antibiotics is associated with blocking bacterial ribosomal RNA and suppressing RNA-dependent protein synthesis. Macrolide resistance rates in S. pneumoniae in the United States currently range from 20 to 40%. The formation of insensitivity to this group of antibiotics may be associated with two independent mechanisms:
The first mechanism is associated with efflux—the ability of a microorganism to actively remove antibiotics from the cell. This mechanism is encoded by the bacterial mef(A) gene and is implemented through the operation of an intracellular pump—the efflux pump. It is characteristic of 14- and 15-membered macrolides; 16-membered macrolides are not subject to efflux. In previous years, efflux led to a slight increase in MIC and a low level of pneumococcal resistance to macrolides. Creating high concentrations of the drug at the site of infection sometimes helped to overcome the work of the efflux pump. However, in recent years, the MICs for S. pneumoniae isolates with the efflux mechanism have increased by another 2–4 times, and this has practically deprived macrolides of the ability to act at the site of infection caused by pathogens resistant to them.
The second mechanism associated with the presence of the erm(B) gene is a modification of the binding region of the antibiotic to its target—23S RNA, a component of the 50S subunit of bacterial ribosomes. The acquisition of this gene by a bacterium immediately leads to the development of a high level of resistance to 14-, 15- and 16-membered macrolides, lincosamides and streptogramin B (MSLB phenotype). The MSLB resistance phenotype of pneumococci is prevalent throughout the world, although in some regions, particularly the United States, the mef(A) gene variant predominates. Isolates have also been described that have both resistance mechanisms at once. Their share has been increasing in recent years, and they are usually resistant to a number of antibiotics.
The only drug from this group that is currently used in clinical practice is clindamycin. The mechanism of its action is due to binding to the 50S ribosomal subunit of the microbial cell and, thus, inhibition of protein synthesis. As a result of the inability to form peptide bonds, the structure of the cell wall is disrupted, which leads to a decrease in the adhesion of S. pneumoniae to host cells and an increase in phagocytic activity towards the bacterium itself. Clindamycin is a bacteriostatic drug, but is bactericidal against some strains of S. pneumoniae. Due to its high activity against streptococci and staphylococci, clindamycin is used to treat secondary community-acquired infections due to S. pneumoniae infection and as an alternative drug in the presence of resistance to beta-lactam antibiotics.
Although lincosamides and macrolides have different chemical structures, they share the same pneumococcal resistance mechanism associated with the erm B gene. Data from the 2008 SENTRY study indicate that only 80% of S. pneumoniae strains in the United States are susceptible to clindamycin . In 2011, this figure was even lower - 78%. Clindamycin is characterized by the phenomenon of inducible resistance that develops during therapy, so it should not be used for empirical treatment of severe pneumococcal infections in the absence of data from a disk diffusion test to suppress the sensitivity of bacteria to clindamycin in the presence of a macrolide.
At the time of the discovery of fluoroquinolones, high sensitivity of S. pneumoniae to these antibiotics was widely observed. However, ciprofloxacin is not currently considered as an antipneumococcal drug, since its pharmacokinetic parameters in therapeutic doses do not interfere with the selection of resistant isolates. In 2007, the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) removed ciprofloxacin and other early fluoroquinolones from their list of drugs for the treatment of infections caused by S. pneumoniae. The latest generation of fluoroquinolones have high antipneumococcal activity, are available in both parenteral and oral forms and play an important role in the treatment of pneumococcal infections resistant to other antibiotics.
Fluoroquinolones directly inhibit bacterial DNA synthesis. For microbial DNA replication, the presence of two enzymes is necessary - DNA gyrase and topoisomerase, which are blocked by the quinolone ring of this group of antibiotics. Individual fluoroquinolones have varying affinities for enzymes involved in replication, with most drugs having greater or lesser affinity for both. The first and second generations of fluoroquinolones act predominantly on topoisomerase II, and subsequent generations act predominantly on topoisomerase IV, which increases their activity against gram-positive bacteria, including S. pneumoniae.
Three mechanisms for the formation of resistance of pneumococci to the action of fluoroquinolones have been described.
The first of them is associated with the accumulation of mutations in the topoisomerase (parC and parE) and DNA gyrase (gyrA and gyrB) genes, leading to structural changes in the active sites of the enzymes. For the development of a high level of resistance to fluoroquinolones of the 3rd–4th generations, a combination of two mutations is required: gyrA and parC. Mutations in the gyrB and parE genes are rare and their clinical significance is unclear.
The second mechanism is associated with the action of the efflux pump, which removes the antibiotic from the bacterial cell. The ability of bacteria to actively remove the antibiotic is different for different fluoroquinolones - for example, levofloxacin is subject to efflux to a much lesser extent than ciprofloxacin.
The third mechanism is plasmid resistance associated with the production of proteins by plasmids that protect the targets of fluoroquinolones. This mechanism is common in the USA, Europe and East Asia. It usually leads to low-level resistance, but can contribute to the development of high-level resistance through cumulative effects when combined with other mechanisms.
The results of the SENTRY study (2008) showed that in North America, 99.4% of S. pneumoniae strains were susceptible to the “new” fluoroquinolones. However, there are also isolated pockets of higher resistance - for example, in Italy, in the period from 2001 to 2004, 5.6% of S. pneumoniae strains were resistant to levofloxacin. Therefore, despite the favorable situation at the moment, vigilance and caution must be maintained when prescribing and using the latest generations of fluoroquinolones in order to preserve them as “reserve drugs”.
Doxycycline, a tetracycline drug, was introduced as an inexpensive empirical treatment for community-acquired pneumonia in the 1940s. By 2002, 16.6% of penicillin-resistant S. pneumoniae were resistant to tetracycline. In recent decades, the number of tetracycline-resistant pneumococcal strains has been increasing everywhere.
The most important mechanism for the formation of resistance of S. pneumoniae to the action of tetracyclines is ribosomal protection, which allows the bacterium to synthesize protein even when the antibiotic binds to the ribosomal target. The mechanism of such protection is not precisely known, but it involves proteins encoded by the tet(O) and tet(M) genes. There are reports that doxycycline is more active than tetracycline against pneumococcal strains carrying the tet(M) gene. This antibiotic can be used to treat pneumococcal infections, but for this, the high sensitivity of S. pneumoniae to it in a given region must be confirmed. It must be taken into account that doxycycline is a broad-spectrum antibiotic, so its use for pneumococcal infections should be limited to avoid the growth of resistance of other bacteria.
The combination of trimethoprim and sulfamethoxazole has been known since 1968. The feasibility of creating such a combination was associated with the synergistic effect of these two drugs on the metabolism of folic acid in bacteria. Sulfamethoxazole blocks the enzyme dihydropteroate synthetase, and trimethoprim blocks dihydrofolate reductase, which are involved in the formation of nucleic acids. Thus, co-trimoxazole prevents the proliferation of bacteria, i.e., it has a bacteriostatic effect.
Initially, the combination was used to treat urinary tract infections, but later the indications for its use expanded to include acute sinusitis, acute otitis media and exacerbation of chronic bronchitis. Due to its low price, co-trimoxazole was included by the WHO in the list of essential drugs. Already in the 1980s, the first cases of S. pneumoniae resistance to trimethoprim/sulfamethoxazole were described. Subsequently, its rapid growth was associated with the very frequent prescription of these drugs for the treatment of acute otitis media in children and the prevention of AIDS in adults - an indication that was considered justified at that time. Trimethoprim and sulfamethoxazole are drugs from different pharmacological groups; there are different mechanisms of bacterial resistance to them, however, since they are used in combination, the corresponding genetic markers in the same strain are usually combined. According to epidemiological studies, 1/3 of S. pneumoniae strains in the United States are resistant to co-trimoxazole, and this figure varies from 25 to 45% in different regions. At the moment, a similar picture is typical for both developed and developing countries. Because of this, co-trimoxazole should not be considered as the drug of choice for the treatment of infections caused by S. pneumoniae.
The spread of S. pneumoniae resistance to most antibiotics in modern medicine has become epidemic. The choice of drug for empirical treatment of pneumococcal infections should be dictated by both the current level of antibiotic resistance and the risk of its further spread in the population. Along with strict control of the use of antibiotics, alternative strategies should be explored to reduce the development of resistance in microorganisms, including the possibility of vaccination.
Detection of the causative agent of streptococcal infection (Streptococcus pneumoniae), during which the genetic material (DNA) of pneumococcus in a sample of biomaterial is determined using the polymerase chain reaction (PCR) method.
Synonyms Russian
Pneumococcus, Weixelbaum diplococcus, Frenkel diplococcus.
English synonyms
Diplococcus pneumoniae.
Research method
Polymerase chain reaction (PCR).
What biomaterial can be used for research?
Venous blood, throat swab (oropharynx), nasopharynx swab, sputum, pleural fluid, cerebrospinal fluid.
How to properly prepare for research?
- It is recommended to drink a large volume of liquid (pure still water) 8-12 hours before collecting sputum.
- 3-4 hours before taking swabs from the oropharynx (throat), do not eat, drink, brush your teeth, rinse your mouth/throat, chew gum, or smoke. 3-4 hours before taking nasal swabs, do not instill drops/sprays or rinse your nose. It is best to take smears in the morning, immediately after a night's sleep.
- Do not smoke for 30 minutes before the test.
General information about the study
Streptococcus pneumoniae (pneumococcus) is a gram-positive diplococcus that is the main causative agent of community-acquired pneumonia. This microorganism can also cause meningitis and sepsis. There is evidence of the role of pneumococcus in the development and progression of bronchial asthma and chronic bronchitis.
People with a reduced immune status are most susceptible to streptococcal pneumonia and meningitis: children, the elderly, HIV-infected people, those suffering from alcoholism and drug addiction.
There are several laboratory methods for isolating pneumococcus: bacteriological (culture of sputum on nutrient media), sputum microscopy and detection of genetic material (DNA) of S. pneumoniae using the polymerase chain reaction method.
Quite often, a sputum sample turns out to be uninformative, and the result of its Gram stain is false negative. The result of the bacteriological method can be obtained only after a few days, which complicates diagnosis and delays the start of specific therapy. Unlike these methods, PCR has high sensitivity and specificity and allows you to obtain an accurate result as soon as possible after delivery of the sample to the laboratory. PCR does not require the presence of a large number of viable microorganisms in a biomaterial sample, since it is not the microorganism itself that is determined, but its genetic material. This advantage makes it possible to establish the etiology of the disease against the background of initiated antibacterial therapy. False positive results are also completely excluded.
Since S. pneumoniae is a representative of the normal microflora of the upper and lower respiratory tract of most people, the diagnosis of pneumococcal pneumonia requires not only the detection of this microorganism in sputum, but also the presence of additional clinical and laboratory signs of an infectious-inflammatory process in the respiratory organs. For example, S. pneumoniae, detected in the sputum of a patient without clinical signs of an infectious lung infection, without abnormalities in a blood test and without changes on an x-ray, should be considered as a normal representative of the microflora of the respiratory tract.
What is the research used for?
- For the diagnosis of infectious (pneumonia, chronic bronchitis) and infectious-allergic lung diseases.
When is the study scheduled?
In the presence of:
- symptoms of typical community-acquired pneumonia (fever, shortness of breath and chest pain, cough with orange-colored sputum (“rusty” sputum);
- symptoms of exacerbation of chronic bronchitis (cough with sputum against the background of increased body temperature, increased sweating, malaise).
What do the results mean?
Reference values: negative.
Positive result:
- pneumococcal pneumonia;
- exacerbation of chronic bronchitis.
Negative result:
- absence of pneumococcal infection.
What can influence the result?
- Most people are asymptomatic carriers of pneumococcus in the upper and lower respiratory tract, so the test result should be interpreted taking into account other clinical and laboratory signs of the disease.