Pharmacological properties of the drug Propafenone
A highly active class I antiarrhythmic drug (according to the Williams classification) with a moderate blocking effect on β-adrenergic receptors. Reduces the maximum depolarization rate and action potential amplitude in Purkinje fibers. Has no effect on resting potential. Effective for ventricular extrasystole, for supraventricular rhythm disturbances, especially paroxysms of atrial fibrillation/flutter. Clinically significant suppression of ventricular tachycardia is observed in at least 50% of patients receiving propafenone. They are also used to relieve and prevent attacks of tachycardia caused by conduction disturbances in the AV node and with WPW syndrome. Propafenone provides a lasting therapeutic effect in 80% of patients with this pathology. Propafenone has been proven effective in treating organic heart lesions, including in patients who have suffered a myocardial infarction. Recommended for resistance to other antiarrhythmic drugs. At doses of 450–1200 mg/day, it eliminates arrhythmia in more than half of these patients. The maximum content in blood plasma is achieved 2–3 hours after oral administration.
Proarrhythmogenic effect of propafenone in patients with atrial flutter.
Yu.V. Zinchenko.
National Scientific Academy of Medical Sciences of Ukraine, Kyiv.
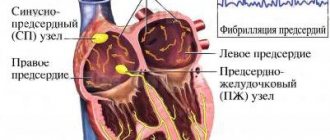
Atrial flutter (AFL) ranks second among tachyarrhythmias in terms of prevalence after atrial fibrillation (AF) with a detection rate of up to 15–20% of all supraventricular rhythm disturbances and is characterized by a regular rhythm with an atrial contraction frequency (AFF) of 250–350 per minute (170– 240 ms). To restore sinus rhythm in AFL, antiarrhythmic drugs (AAP), electrical pulse therapy, cardiac pacing, and catheter ablation are used [11]. The optimal and most effective method of restoring sinus rhythm is transesophageal pacing (TEPS), the effectiveness of which reaches 75–98%. Electrical stimulation of the atria is carried out with a frequency exceeding the heart rate in order to penetrate the impulse into the macroentry circle and create conditions for its interruption [2, 4, 9].
The pathophysiology of AFL is based on the correct atrial circuit of excitation circulation. This circuit includes an electrical excitation impulse and a section of the atrium myocardium ready for conduction. Class IA AAPs reduce the conduction speed in the re-entry circuit and generally shorten the period of excitability. Class IC AAPs slow down impulse conduction and thereby reduce FSP. In contrast, class III AAPs (amiodarone) increase the refractory period and can stop AFL, since the impulse encounters refractory tissue. Electrical stimulation of the atria can stop atrial fibrillation by creating a functional block in one of the directions of the re-entry circle. In addition, the effectiveness of stimulation can be increased by antiarrhythmic therapy (AAT), which facilitates the creation of a zone of absolute refractoriness [4, 6, 16].
It is known that in addition to antiarrhythmic, almost all AAPs have a proarrhythmic effect [5]. Proarrhythmia is the worsening of the course or increase in the frequency of previously existing arrhythmias, or the appearance of new ones (actually a proarrhythmogenic effect) as a result of the administration of AAP. In most cases, the proarrhythmogenic effect is dose-dependent. It is observed in approximately 13% of cases of AAP prescription.
Mechanisms of proarrhythmias
Almost all AAPs, under certain circumstances, can have a proarrhythmogenic effect, the appearance of which is facilitated by:
- a decrease or increase in the serum concentration of AAP due to its interaction with other drugs in renal or hepatic failure or other pharmacokinetic disorders;
- idiosyncrasy to AAP;
- hypokalemia or hypomagnesemia;
- interaction between AAP and the autonomic nervous system;
- impact of AAP on myocardial contractility and peripheral vascular bed.
The mechanisms of the proarrhythmogenic action of AAP and its electrophysiological basis have not yet been sufficiently studied. However, knowledge of the mechanisms of antiarrhythmic action of drugs allows us to understand and predict the specific proarrhythmic effect of specific drugs. For example, class IA drugs, by slowing down conduction through the conduction system of the heart and the atrial myocardium, can contribute to the occurrence of re-entry. Beta-blockers, by preventing the acceleration of myocardial conduction under the influence of catecholamines, can increase differences in conduction velocity across different parts of the myocardium and also contribute to the occurrence of re-entry loops.
The antiarrhythmic effect in tissues with a short refractory period is achieved by reducing the dispersion of refractory periods and the window of excitability. Sodium channel blockers, in addition to reducing the variance of refractory periods, may also slow conduction, increase the incidence of ventricular tachycardia (VT), slow ventricular tachycardia (VT) waves with 1:1 conduction, and increase the incidence of sudden death, especially in patients with coronary artery disease. Drugs that prolong the action potential increase the incidence of spindle-shaped ventricular tachycardias (torsades de pointes), especially against the background of bradyarrhythmia and hypokalemia.
The proarrhythmogenic effect may also be due to an increase in normal or abnormal automaticity or the formation of early (quinidine) or late (cardiac glycosides) afterpotentials. Prolongation of repolarization and the duration of the effective refractory period under the influence of AAP increases the blockade of the propagation of the premature impulse, which can appear during phase 3 of repolarization, spreading slowly and abnormally. Class IA drugs have a vagolytic effect and therefore can cause a paradoxical increase in heart rate (HR), up to the development of ventricular fibrillation (VF). In other words, we can say that almost any AAP under certain conditions can produce a proarrhythmogenic effect [5, 6].
Most often, the proarrhythmogenic effect of AAP occurs in patients with severe rhythm disturbances due to coronary heart disease complicated by circulatory failure, that is, in patients with hypoxic-dystrophic changes in the myocardium, leading to its electrical instability. Under such circumstances, AAPs, as a rule, contribute to the development of uneven repolarization and increased dispersion of myocardial refractoriness. Other common factors that determine the proarrhythmic effect of AAP are catecholamines that induce trigger activity (as, for example, during therapy with cardiac glycosides, procainamide). Another common arrhythmogenic mechanism is associated with vagolytic reactions caused by certain drugs. The classic drug of this type is disopyramide.
Diagnostics
To assess the proarrhythmogenic effect, it is necessary to use electrocardiography, Holter and telemetric ECG monitoring, electrophysiological studies (EPS), and stress tests. When using AAP (especially class I) in severely ill patients, it is necessary to monitor the heart rhythm throughout the entire period of AAP use.
Clinical predictors of a possible proarrhythmic effect of any used AAP may be:
- left ventricular (LV) ejection fraction (EF) less than 40%;
- the presence of a LV aneurysm even in the absence of global systolic dysfunction;
- presence of symptoms of heart failure (HF);
- changes in blood pH, electrolyte disorders;
- autonomic imbalance with decreased heart rate variability;
- the presence of late potentials on a high-resolution ECG;
- hormonal disorders;
- the presence of existing episodes of stable VT or VF;
- easier occurrence of arrhythmias during physical activity.
Timely EPI allows the earliest detection of the proarrhythmic effect of AAP. The following criteria can be used as criteria for the presence of an arrhythmogenic effect:
- easier induction of arrhythmia with stimulation compared with placebo;
- transition of short paroxysms into persistent ones at the same or lower frequency of stimulation;
- increased frequency of ventricular extrasystoles during cardiac pacing.
In the 1970s and 1980s, the development of AAPs was primarily aimed at treating ventricular arrhythmias as markers of sudden death. The 1989 CAST (Cardiac Arrhythmia Suppression Trial) changed these attitudes. In the last decade, the main efforts have been aimed at finding drugs for the treatment of AF and AFL.
Classification of proarrhythmias
Types of proarrhythmias in the treatment of AF or AFL with various AAPs according to the classification of E.M. Vaughan Williams [10]:
1. Ventricular proarrhythmias:
- torsades de pointes (IA and III class),
- persistent monomorphic VT (IC class),
- persistent polymorphic VT/VF without QT prolongation (IA, IC and III class).
2. Atrial proarrhythmias:
- relief of provocation of relapses (IA, IC, and III class),
- transformation of AF into TP (IC class),
- increasing the defibrillation threshold (IC class).
3. Impaired conduction or formation of impulses:
- acceleration of ventricular contraction frequency (VFR) in AF (class IA and IC),
- acceleration of conduction along the accessory pathway (atrioventricular conduction blockers),
- sinus node dysfunction, atrioventricular block (almost all AAP).
Proarrhythmogenic effects of class IC AAP
For class I AAPs, the main target of action is sodium channels. AAP molecules penetrate into their lumen from the inner surface of the membrane and are fixed on the receptors. This leads to a slowdown in the spread of excitation through the conduction system of the heart and myocardium. At therapeutic concentrations, class I AAPs often cause an increase in the duration of the QRS complex and the PR interval. First of all, this concerns class IC AAPs - powerful sodium channel blockers. Class IA AAPs have a lesser effect on the QRS complex, and class IB AAPs have a very weak effect. The effect of AAPs of the last two groups on the duration of the PR interval is practically not expressed at all: in class IA AAPs - due to the anticholinergic (atropine-like) properties of these drugs, and in class IB AAPs - due to a weak effect on conduction in the atria and atrioventricular node [6 ].
The rare development of VT of the torsades de pointes type when using class IB drugs is associated with the fact that they do not block the outgoing potassium current, and class IC AAPs block only the delayed potassium current. Propafenone slows down the conduction velocity in the atria and ventricles in the myocardium with a slight increase in repolarization time, which is why it belongs to class IC. However, in some patients it may cause non-selective blockade of potassium channels, prolonging repolarization and the QT interval. In some patients, the background sodium current and the outgoing potassium current may have different sensitivities to propafenone, resulting in occasional QT prolongation and torsade de pointes VT. In addition, it must be remembered that treatment of AF with class IC drugs can induce AF in 3.5–5% of cases using a 1:1 ratio.
Propafenone is an effective AAP in patients with AF, allowing restoration of sinus rhythm both intravenously and when administered orally. Pharmacokinetics allows it to be administered orally as a loading dose. In the 90s of the last century, several randomized studies were carried out, which demonstrated its high effectiveness in patients with recent paroxysms of AF–AFL when administered in a loading dose orally [1, 3, 7, 8, 15].
According to some studies, the incidence of various proarrhythmogenic side effects after taking propafenone is no higher than after taking placebo. In one study, AFL or other tachycardia lasting at least 1 minute occurred in 7% of patients treated with propafenone and in 6% of patients treated with placebo. In another study, AFL with 1:1 atrioventricular conduction and a heart rate of more than 150 per minute occurred with equal frequency in the groups of patients receiving propafenone and placebo (14%) [10].
In case of AFL, the most effective method of restoring sinus rhythm is TEE [2, 9]. According to a number of studies, there has been an increase in the effectiveness of TEE in restoring sinus rhythm in patients with paroxysms of atrial flutter against the background of preliminary use of class IC AAP – propafenone [12–14].
In this regard, the case of the dose-dependent proarrhythmogenic effect of the class IC AAP, propafenone, during the restoration of sinus rhythm in a patient with prolonged paroxysm of type I AFL is of interest.
Patient B., 53 years old, went to the institute’s clinic with a diagnosis of stage II hypertension. Myocardiofibrosis. Persistent form of TP. Stage I heart failure.
Increased blood pressure (BP) for many years, was not examined, was not treated. Paroxysm of TP occurred for the first time, lasting 40 days. From the anamnesis it is known that the patient has diffuse goiter of the 2nd degree, clinical euthyroidism.
Objectively: Hypersthenic physique, increased nutrition. In the lungs, breathing is vesicular. Blood pressure 140/90 mm Hg. Art. The activity of the heart is rhythmic, the sounds are muffled. There is no peripheral edema. ECG: AFL with an average heart rate of 120 per minute, a heart rate of 240 ms and a conduction ratio to the ventricles of 2:1. Complete right bundle branch block (RBBB).
The structural and functional state of the myocardium was assessed using a Sonoline-Omnia echocardiograph (Siemens, Germany) with a sensor frequency of 2.5 MHz. In two-dimensional and M-mode, linear and volumetric characteristics of the left (LA) and right (RA) atria and the left (LV) and right (RV) ventricles were determined. Echocardiography protocol: LA size (M-mode) – 41 mm; S LA (diastole) – 22.8 cm2; S LA (systole) – 26.9 cm2; V LA (diastole) – 73 ml; V LA (systole) – 99 ml; LA EF (according to Simpson) – 26%; LV end-systolic size – 38 mm; LV end-diastolic size (EDD) – 49 mm; LV end-systolic volume – 57 ml, LV end-diastolic volume – 111 ml, LV EF – 48%, LV interventricular septum thickness – 11.5 mm, LV posterior wall thickness – 10 mm, RV EDV (M-mode) – 29 mm, S PP (diastole) – 20.6 cm2, S PP (systole) – 23.6 cm2, V PP (diastole) – 61 ml, V PP (systole) (according to Simpson) – 74 ml, PP EF – 18%, index LV myocardial mass – 90.1 g/m2.
The patient also underwent transesophageal echocardiography: no blood clots were detected. After the examination, propafenone was prescribed at a dose of 450 mg/day. After selecting the target dose of the anticoagulant (phenyline), the patient underwent therapeutic TEE on an outpatient basis.
On the ECG (05/19/2009) before stimulation: TP with an average heart rate of 115 per 1 min, heart rate of 270 ms and conduction ratio to the ventricles 2:1, 3:1, QRS – 200 ms, PBPBB.
TEPS was carried out using a temporary pacemaker Cordelectro-05 (Lithuania), a diagnostic electrode "PEDM-6" (Ukraine); ECG recording was carried out using a Mingograf-82 electrocardiograph (Siemens-Elema, Sweden). Stimulation was started at a frequency 25–35% higher than the frequency of atrial fibrillation, and was subsequently increased until the transition to persistent atrial fibrillation. Initial stimulation parameters: current strength - 1.5 mA, pulse duration - 10 ms, stimulation duration - 2-3 s, interpolar intervals - 10 mm.
After the TEE protocol (current intensity – 2 mA, number of stimulation bursts – 25), AFL was transferred to persistent AF. After 20 minutes, 1000 mg of procainamide was injected intravenously. The ECG shows a widening of the QRS complex, but sinus rhythm has not been restored. It is recommended to continue taking propafenone, but at a dose of 900 mg/day, with the aim of possible drug cardioversion.
Within 24 hours, a reverse transformation of AF into atrial flutter occurred, but the patient was recommended to continue taking the drug. During treatment, the patient’s condition worsened – shortness of breath increased. ECG (05/22/2009): AFL with an average heart rate of 110 per minute, heart rate of 290 ms and conduction ratio to the ventricles 1:1, 2:1, 3:1, QRS – 240 ms, PBPBB.
Repeated TEE was performed. Stimulation protocol: there was a deterioration in the imposition of rhythm on the atria: the frequency and duration of stimulation were repeatedly increased (up to 10 s), the pulse amplitude and interpolar distance were increased to 20 mm, and the location of the electrode was changed. More than 80 bursts of stimulation were performed with a gradually increasing frequency, and only with a current strength of 5 mA was it possible to convert AFL into AF. The patient continued taking propafenone at the same dose. The next day, the ECG shows a pattern of TP with increasing periods of the ventricular conduction ratio of 1:1.
Considering the ineffectiveness of repeated stimulation against the background of propafenone at a dose of 450 and 900 mg/day; the appearance of its proarrhythmogenic effect: increasing the threshold of myocardial excitability, improving atrioventricular conduction with the appearance of 1:1 periods; the need for a more aggressive pacing protocol, as well as clinical worsening of the arrhythmia, the AAP was discontinued.
On the third day, the patient underwent cardiac pacing without AAT. ECG (05.26.2009): AFL with an average heart rate of 120 per minute, heart rate of 240 ms and conduction ratio to the ventricles of 2:1, QRS – 180 ms, RBBB. Blood pressure 120/100 mm Hg. Art. After five bursts of stimulation with a current amplitude of 1.5 mA, the AF was transferred to the AF. After 15 minutes, the patient regained sinus rhythm with a frequency of 60 contractions per minute.
After successful cardioversion, AAT was not performed. A week later, the patient maintains sinus rhythm - 65 contractions per 1 minute, on the ECG - PBPBB. Holter ECG monitoring was performed: average heart rate per day – 65 per 1 min (min. – 43, max. – 98); supraventricular extrasystole – 189, ventricular – 6; No paroxysmal rhythm disturbances were detected. After 24-hour blood pressure monitoring, the patient was prescribed antihypertensive treatment (candesartan 16 mg/day).
During the observation period of 6 months, no recurrences of arrhythmia were recorded. Thus, we successfully restored the rhythm in a patient with a long-term (more than 45 days) paroxysm of atrial flutter without antiarrhythmic preparation.
The presented clinical case showed a dose-dependent proarrhythmogenic effect of propafenone, which was manifested by an increase in the threshold of excitability of the atrial myocardium and, thereby, difficulty in the possibility of an electrical impulse entering the vulnerable window of the re-entry loop, as well as an improvement in atrioventricular conduction with the appearance of 1:1 conduction periods to the ventricles, the need application of a more aggressive stimulation protocol: increasing the number of bursts and duration of stimulation; the use of greater current strength of stimulating impulses necessary to impose an artificial rhythm on the atria; increasing the interelectrode distance of the stimulating electrode to 20 mm. In addition, it should be noted that the reverse transformation of AF into AFL while taking propafenone is also due to the proarrhythmogenic effect of AAP, with the formation of stability of the re-entry circle.
The presented ECG of the patient shows no significant dynamics, except for the appearance of periods of 1:1 atrioventricular conduction while taking propafenone at a dose of 900 mg/day. The increase in the duration of the QRS complex is associated with the direct electrophysiological effect of the drug.
Thus, the interest of this clinical case is that, despite the generally accepted opinion that the effectiveness of rhythm restoration increases against the background of preliminary antiarrhythmic preparation, there are cases when AAT has proarrhythmogenic effects that cannot be predicted by a surface ECG and which appear only when the rhythm is restored .
According to some studies, it is possible to restore the rhythm in patients with prolonged paroxysms of atrial flutter without preliminary AAT [2]. In the EPI laboratory of the AR department, for many years, TEE has been performed on an outpatient basis for AFL, in patients without severe cardiac pathology, severe heart failure and rare (less than one episode every 3 months) paroxysms, without prior antiarrhythmic preparation. The duration of paroxysm in these cases does not affect the tactics of rhythm restoration. It should be noted that patients with paroxysms of atrial flutter for more than 7 days undergo transesophageal echocardiography to exclude blood clots and predictors of thrombus formation in the atrium and left atrium, as well as to identify extracardiac factors of thrombus formation. Before restoring the rhythm, all patients undergo treatment of the underlying disease, correction of blood pressure and compensation of heart failure.
Consequently, doctors performing cardiac pacing in patients with AFL must remember that cases of ineffective imposition of an artificial rhythm on the atria and the stability of the re-entry circle can be caused not only by anatomical, structural-functional and electrophysiological mechanisms, but also by the proarrhythmogenic effect of AAP.
Literature
- Bunin A.Yu., Anfalova L.K. The effectiveness of propafenone in the oral relief of paroxysms of atrial fibrillation. Placebo-controlled study // Ros. cardiol. magazine – 2005. – No. 6. – P. 57-61.
- Zinchenko Yu.V., Valizade Chari Jafar, Stepanenko A.P. and others. Antiarrhythmic preparation before restoration of sinus rhythm in patients with paroxysms of atrial flutter for more than 7 days // Ukr. cardiol. magazine – 2009. – No. 3. – P. 72-78.
- Isakova N.N., Kulakov Yu.V., Kononova A.M. et al. Efficiency of treatment of paroxysmal atrial fibrillation with propafenone and amiodarone // Ros. cardiol. magazine – 2006. – No. 3. – P. 58-62.
- Kushakovsky M.S. Cardiac arrhythmias. Heart rhythm and conduction disorders: A guide for doctors. – St. Petersburg, Foliot, 2004. – 672 p.
- Nikonov V.V., Kinoshenko E.I., Grushko T.I. Complications of antiarrhythmic therapy // Emergency Medicine. – 2009. – No. 1. – T. 20. – P. 9-18.
- Podlesov A.M., Boytsov S.A., Egorov D.F. and others. Atrial fibrillation. – St. Petersburg: ELBI-SPb., 2001. – 203 p.
- Preobrazhensky D.V., Sidorenko B.A., Kiktev V.G. and others. Propafenone: basics of clinical pharmacology and place in the treatment of atrial fibrillation // Klin. gerontology. – 2005. – T. 11. – No. 11. – P. 56-65.
- Skibitsky V.V., Kudryashov E.A., Fendrikova A.V. and others. Propafenone in complex anti-relapse therapy of persistent atrial fibrillation // Ros. cardiol. magazine – 2007. – No. 4. – P. 44-47.
- Chireikin L.V., Shubik Yu.V., Medvedev M.M. and others. Methods of transesophageal electrocardiography and cardiac pacing. – St. Petersburg: Inkart, 1999. – 150 p.
- ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation // Circulaition. – 2006. – Vol. 114. – P. 257-354.
- ACC/AHA/ESC 2003 Guidelines for the management of patients with supraventricular arrhythmias // Eur. Heart. J. – 2003. – Vol. 24. – No. 20. – R. 1857-1897.
- Doni F., Manfredi M., Piemonti C. et al. New onset atrial flutter termination by overdrive transoesophageal pacing: effects of different protocols of stimulation // Europace. – 2000. – No. 2. – R. 292-296.
- Doni F., Della Bella P., Kheir A. et al. Atrial flutter termination by overdrive transesophageal pacing and the facilitating effect of oral propafenone // Amer. J. Cardiology. – 1995. – No. 76. – R. 1243-1246.
- Doni F., Staffiere E., Manfredi M. et al. Type II atrial flutter interruption with transesophageal pacing: use of propafenone and possible change of the substrate // Pacing Clin. Electrophysiol. – 1996. – No. 19. – R. 1958-1961.
- Sun JL, Guo JH, Zhang N. et al. Clinical comparison of ibutilide and propafenone for converting atrial flutter // Cardiovasc. Drugs Ther. – 2005. – No. 19. – R. 57-64.
- Waldo A.L. Atrial flutter: from mechanism to treatment // Armonk, NY: Future Publishing Company. – 2001. – 64 p.
Ukrkardio
Use of the drug Propafenone
Individual dose selection is carried out under the control of ECG and blood pressure. QRS and the QT increases by more than 20%, it is necessary to reduce the dose or temporarily discontinue propafenone until the ECG values normalize. The therapeutic dose for patients weighing 70 kg is usually 450–600 mg/day (150 mg 3 times a day or 300 mg 2 times a day). In some cases, it can be increased to 900 mg (300 mg 3 times a day). As an exception, it can be prescribed at a higher dose under strict ECG monitoring. Patients with lower body weight should reduce doses accordingly. In elderly patients and patients with severe myocardial damage at the beginning of treatment, the dose should be increased gradually.
Propafenone 3.5 mg/ml 10 ml 10 pcs. injection
pharmachologic effect
The effect is based on a local anesthetic and direct membrane-stabilizing effect on myocardiocytes. It has m-anticholinergic and beta-adrenergic blocking (weak) effects, blocks sodium channels.
Composition and release form Propafenone 3.5 mg/ml 10 ml 10 pcs. injection
film-coated tablets - 1 tablet. propafenone hydrochloride - 150 mg excipients: lactose monohydrate; MCC; sodium lauryl sulfate; sodium starch glycolate; povidone; talc; magnesium stearate; opadry white Y-1-7000. in a dark glass bottle 40 pcs.; 1 bottle in a cardboard pack.
Description of the dosage form
White, round, biconvex, film-coated tablets, scored on one side.
Directions for use and doses
For the treatment and prevention of extrasystole, it is prescribed orally at an initial dose of 450-600 mg per day. If the effect is insufficient, the dose is increased to 900 mg. The tablets should be taken after meals, without chewing, with a small amount of liquid. For paroxysmal rhythm disturbances, it is prescribed intravenously by drip at an initial dose of 500 mcg per kg of body weight, if the effect is insufficient - 1-2 mg/kg.
Pharmacodynamics
Class IC antiarrhythmic drug. Blocks fast sodium channels, causes a dose-dependent decrease in the rate of depolarization, inhibits phase 0 of the action potential, its amplitude in Purkinje fibers and contractile fibers of the ventricles, and inhibits automaticity. Extends conduction time through the sinoatrial node (SA) and atria, slows down conduction through Purkinje fibers. Causes prolongation of the PQ interval and expansion of the QRS complex, as well as the AH and HV intervals. By slowing down conduction, it lengthens the effective refractory period in the atria, in the AV node, in accessory bundles and, to a lesser extent, in the ventricles. The QT interval does not change significantly. Electrophysiological effects are more pronounced in ischemic compared with normal myocardium. The local anesthetic effect approximately corresponds to the activity of procaine. Beta-blocking activity corresponds to approximately 1/40 of the activity of propranolol. It has a negative inotropic effect, which usually occurs when the left ventricular ejection fraction decreases below 40%. The action begins 30 minutes after oral administration, reaches a maximum after 2–3 hours and lasts 8–12 hours.
Pharmacokinetics
After oral administration, absorption is more than 95%. Bioavailability increases nonlinearly with increasing dose: when increasing a single dose from 150 to 300 mg, it increases from 5 to 12%, and up to 450 mg, to 40-50%. Cmax fluctuates in the range of 500-1500 µg/l, Tmax - 2-3 hours. Passage through the BBB and the placental barrier is insignificant. Distribution volume - 3-4 l. Communication with proteins of plasma and internal organs (liver, lungs, etc.) - 85-97%. Metabolized almost completely. 11 metabolites of the drug have been described, of which the metabolically active ones are 5-hydroxypropafenone and N-depropylpropafenone, which have antiarrhythmic activity comparable to propafenone. Oxidative metabolism depends on a specific cytochrome, whose activity is genetically determined. The therapeutic range of plasma concentration is 0.5-2.0 mg/l. T1/2 in persons with fast metabolism is 2-10 hours, slow - 10-32 hours, biological T1/2 - 6.2 hours. Excreted by the kidneys.
Indications for use Propafenone 3.5 mg/ml 10 ml 10 pcs. injection
- supraventricular and ventricular extrasystoles;
- supraventricular tachycardia, ventricular tachycardia.
Contraindications
- severe forms of chronic heart failure;
- electrolyte imbalance;
- myasthenia gravis;
- bronchial asthma;
- hypersensitivity to the drug.
Application Propafenone 3.5 mg/ml 10 ml 10 pcs. solution for injection during pregnancy and lactation
Contraindicated during pregnancy, breastfeeding and childhood.
special instructions
Treatment, especially the beginning of the course, must be carried out under constant monitoring of ECG and blood electrolyte balance (especially potassium concentration). It is necessary to start taking the drug in a hospital setting, due to the increased risk of proarrhythmogenic effects. The activity of “liver” transaminases should be periodically determined. In patients with insufficient liver function, bioavailability increases by 70% (cumulation is possible), so it is recommended to reduce the dose and regularly monitor laboratory parameters. In patients with a pacemaker, the dose must be determined with extreme caution. Patients taking anticoagulants and hypoglycemic drugs for a long time require careful clinical and laboratory monitoring. If SA or AV block of the third degree or frequently repeated extrasystole appears during therapy, treatment must be interrupted. It is recommended to use the drug only as directed and under the supervision of a physician, given its likely proarrhythmogenic effect. During the treatment period, it is necessary to refrain from driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Overdose
Symptoms: persistent decrease in blood pressure, nausea, dry mouth, vomiting, mydriasis, drowsiness, extrapyramidal disorders, confusion, bradycardia, prolongation of the QT interval, impaired intraatrial and intraventricular conduction, SA and AV blockade, ventricular tachyarrhythmias, paroxysms of polymorphic ventricular tachycardia, asystole , coma, convulsions, delirium, pulmonary edema (symptoms of intoxication can occur with a single dose of 2 times the daily dose, and appear after an hour, a maximum of several hours). Treatment: gastric lavage, defibrillation, administration of dobutamine, diazepam; if necessary, mechanical ventilation and indirect cardiac massage. Hemodialysis is ineffective.
Side effects Propafenone 3.5 mg/ml 10 ml 10 pcs. injection
- nausea, feeling of heaviness in the stomach, constipation;
- headache, dizziness;
- skin rashes;
- liver dysfunction;
- blood picture disturbance;
- decrease in sperm count.
Drug interactions
Combination with lidocaine is contraindicated (the cardiodepressive effect increases). Increases the plasma concentration of propranolol, indirect anticoagulants, cyclosporine. Enhances the effect of warfarin (blocks metabolism). When used simultaneously with beta-blockers and tricyclic antidepressants, the antiarrhythmic effect may be enhanced; with local anesthetics, the risk of central nervous system damage may increase. Cimetidine and quinidine, by slowing metabolism, increase the concentration of propafenone in plasma by 20%, rifampicin decreases it. Amiodarone increases the risk of developing pirouette tachycardia. Drugs that suppress the SA and AV nodes and have a negative inotropic effect increase the risk of side effects. Drugs that inhibit bone marrow hematopoiesis increase the risk of myelosuppression.
Side effects of the drug Propafenone
Rarely observed. When taking propafenone in high doses, anorexia, a feeling of fullness in the stomach, nausea, vomiting, a bitter taste and a feeling of numbness in the mouth may occur; in isolated cases, decreased visual acuity, headache, anxiety, fear, sleep disturbance, confusion, and extrapyramidal symptoms were observed. Skin manifestations of allergic reactions are sometimes observed. In elderly patients, orthostatic disorders may occur, in rare cases - bradycardia, conduction disturbances of the sinus and AV nodes; The severity of heart failure may also increase. In rare cases, cholestasis is observed, which is independent of the dose and disappears after discontinuation of the drug. Taking propafenone in high doses can cause a decrease in potency. Leukopenia, granulocytopenia, and thrombocytopenia are very rarely observed. The composition of peripheral blood is normalized after discontinuation of propafenone.
Drug interactions Propafenone
When using local anesthetics simultaneously, as well as drugs that reduce heart rate and inhibit myocardial contractility (beta-adrenergic receptor blockers, tricyclic antidepressants), it is necessary to take into account the possibility of mutually enhancing the effect. Cases of increased plasma levels of propranolol, metoprolol and digoxin with simultaneous use of propafenone have been described. If signs of overdose appear, the content of drugs in the blood plasma should be monitored and, if necessary, their doses should be reduced. There are reports of an increase in the level of propafenone in the blood when it is used simultaneously with cimetidine, an increase in prothrombin time when combined with indirect anticoagulants.