What do drugs have in common?
To understand what the difference is and which drug is better - Rosuvastatin or Atorvastatin, it is necessary to conduct a comparative analysis. Both drugs under consideration are representatives of the new generation of statins. It is necessary to select one that, along with a noticeable therapeutic effect, has minimal side effects.
Their mechanism of action is approximately the same, but there are slight differences. If you understand the difference between them, you can adjust the treatment of a particular patient, which will significantly improve the prognosis for his recovery. The commonality between Rosuvastatin and Atorvastatin is that both drugs have a dual effect - they reduce the level of bad cholesterol and increase the content of good cholesterol.
Also common to these two drugs is that they:
- improve the condition of the inner lining of blood vessels during its dysfunction;
- improve blood flow through vessels;
- have a beneficial effect on the vascular wall.
Both drugs have common indications for use. Since they belong to the latest generation of statins, they can be prescribed not only for the treatment of diseases, but also for their prevention, which was difficult with the first and second generation statins due to the many side effects from them.
Therefore, the drugs in question are recommended for use in patients who have an increased risk of developing coronary heart disease. Factors that increase this risk include:
- age over 55 years;
- a history of diabetes mellitus;
- addiction to smoking;
- high blood pressure;
- hereditary predisposition to high cholesterol;
- reduced levels of high-density lipoproteins in the blood.
Atorvastatin and Rosuvastatin are not prescribed for the treatment of children and adolescents, during breastfeeding and pregnancy, or in the acute stage of liver disease. They are used with caution if the patient suffers from alcoholism, is predisposed to myopathy, or has a history of renal failure.
The maximum therapeutic effect from taking new generation statins is achieved after 4 weeks from the start of treatment. If the medicine is taken by a woman who has retained reproductive capabilities, then she is recommended to take care of reliable contraception along with statins.
Both drugs have minimal side effects and are therefore well tolerated by patients. They can be taken regardless of food intake and without reference to a specific time of day.
High levels of “bad” cholesterol lead to blockage of blood vessels
Rosuvastatin
Renal dysfunction
In patients receiving high doses of rosuvastatin (particularly 40 mg/day), tubular proteinuria was observed, which was detected using test strips and in most cases was intermittent or short-term. Such proteinuria does not indicate acute illness or progression of concomitant renal disease. The incidence of serious renal dysfunction observed in post-marketing studies of rosuvastatin is higher when taking a dose of 40 mg/day. In patients taking Rosuvastatin at a dose of 30 or 40 mg/day, it is recommended to monitor renal function during treatment (at least once every 3 months).
Effect on the musculoskeletal system
The following musculoskeletal effects have been reported with rosuvastatin at all doses, but particularly at doses greater than 20 mg/day: myalgia, myopathy, and in rare cases, rhabdomyolysis. Very rare cases of rhabdomyolysis have been reported with the simultaneous use of HMG-CoA reductase inhibitors and ezetimibe. This combination should be used with caution, as pharmacodynamic interactions cannot be excluded.
As with other HMG-CoA reductase inhibitors, the incidence of rhabdomyolysis with post-marketing use of rosuvastatin is higher when using a dose of 40 mg/day.
Determination of serum CPK activity
Serum CPK activity cannot be determined after intense physical exercise and in the presence of other possible reasons for an increase in its activity; this may lead to incorrect interpretation of the results obtained. If the initial serum CPK activity is significantly exceeded (5 times higher than the upper limit of normal), a repeat analysis should be performed after 5-7 days. Therapy should not be started if the results of a repeat analysis confirm the initial high serum CPK activity (more than 5 times the upper limit of normal).
Before starting therapy
Depending on the daily dose, Rosuvastatin should be administered with caution to patients with existing risk factors for myopathy/rhabdomyolysis.
These factors include:
- renal dysfunction,
- hypothyroidism,
- history of muscle diseases (including family history),
- history of myotoxic effects when taking other HMG-CoA reductase inhibitors or fibrates,
- excessive alcohol consumption,
- age over 65 years,
- conditions in which the concentration of rosuvastatin in the blood plasma may increase,
- simultaneous use of fibrates.
In such patients, it is necessary to evaluate the risks and possible benefits of therapy. Clinical monitoring is also recommended. If the initial serum CK activity is higher than 5 times the upper limit of normal, therapy with Rosuvastatin cannot be started.
During drug therapy
The patient should be informed to immediately report to the doctor if muscle pain, muscle weakness or spasms occur unexpectedly, especially in combination with malaise and fever. In such patients, serum CPK activity should be determined. Therapy should be discontinued if serum CPK activity is significantly increased (more than 5 times the upper limit of normal), or if muscle symptoms are severe and cause daily discomfort (even if serum CPK activity is no more than 5 times the upper limit of normal). exceeds the upper limit of normal). If symptoms disappear and serum CPK activity returns to normal, resumption of use of Rosuvastatin or other HMG-CoA reductase inhibitors in lower doses should be considered under close medical supervision. Monitoring serum CPK activity in the absence of symptoms is impractical.
Very rare cases of immune-mediated necrotizing myopathy have been reported with clinical manifestations in the form of persistent weakness of the proximal muscles and increased CPK activity in the blood serum during therapy or upon discontinuation of the use of HMG-CoA reductase inhibitors, including rosuvastatin. Additional studies of the muscular and nervous system, serological studies, and therapy with immunosuppressive drugs may be required. There were no signs of increased effects on skeletal muscles when taking rosuvastatin and concomitant therapy. However, an increase in the number of cases of myositis and myopathy has been reported in patients taking other HMG-CoA reductase inhibitors in combination with fibric acid derivatives (for example, gemfibrozil), cyclosporine, nicotinic acid in lipid-lowering doses (more than 1 g / day), antifungals - azole derivatives, HIV protease inhibitors and macrolide antibiotics.
When used simultaneously with certain HMG-CoA reductase inhibitors, gemfibrozil increases the risk of developing myopathy. Thus, the simultaneous use of Rosuvastatin and gemfibrozil is not recommended. The benefits of further changes in plasma lipid concentrations when using the drug Rosuvastatin in combination with fibrates or nicotinic acid in lipid-lowering doses must be carefully weighed against the possible risks. The drug Rosuvastatin at a dose of 40 mg/day is contraindicated for combination therapy with fibrates.
Rosuvastatin should not be used simultaneously or within 7 days after discontinuation of systemic fusidic acid therapy. In patients in whom the use of fusidic acid is considered necessary, statin therapy should be discontinued for the entire duration of fusidic acid therapy. There have been reports of rhabdomyolysis (including death in some cases) in patients receiving fusidic acid concomitantly with statins. The patient should seek immediate medical attention if any symptoms of muscle weakness, pain, or tenderness occur.
Therapy with Rosuvastatin can be resumed 7 days after the last dose of fusidic acid.
In exceptional cases, when long-term use of systemic fusidic acid is required, for example, in the treatment of severe infections, the need for simultaneous use of Rosuvastatin and fusidic acid should be considered individually and subject to careful medical supervision.
Due to the increased risk of rhabdomyolysis, Rosuvastatin should not be used in patients with acute conditions that may lead to myopathy or conditions predisposing to the development of renal failure (for example, sepsis, hypotension, major surgery, trauma, severe metabolic, endocrine or electrolyte disturbances , uncontrollable seizures).
2-4 weeks after the start of treatment and/or when the dose of Rosuvastatin is increased, monitoring of lipid metabolism parameters is necessary (dose adjustment is required if necessary).
Liver
Depending on the daily dose, Rosuvastatin should be used with caution in patients with excessive alcohol consumption and/or in patients with a history of liver disease, or its use is contraindicated (see sections "Contraindications" and "Precautions").
It is recommended to determine liver function tests before the start of therapy and 3 months after its start. The use of the drug Rosuvastatin should be discontinued or the dose of the drug should be reduced if the activity of “liver” transaminases in the blood serum is 3 times higher than the upper limit of normal.
In patients with hypercholesterolemia due to hypothyroidism or nephrotic syndrome, underlying diseases should be treated before starting treatment with Rosuvastatin.
Ethnic characteristics
During pharmacokinetic studies, an increase in the plasma concentration of rosuvastatin was noted in representatives of the Mongoloid race compared to representatives of the Caucasian race.
Interstitial lung disease
Isolated cases of interstitial lung disease have been reported with the use of certain HMG-CoA reductase inhibitors, especially over long periods of time. Manifestations of the disease may include shortness of breath, non-productive cough and deterioration in general health (weakness, weight loss and fever).
If interstitial lung disease is suspected, therapy with HMG-CoA reductase inhibitors should be discontinued.
Diabetes mellitus type 2
In patients with glucose concentrations between 5.6 and 6.9 mmol/L, rosuvastatin therapy was associated with an increased risk of developing type 2 diabetes mellitus.
HIV protease inhibitors
Concomitant use of the drug with HIV protease inhibitors is not recommended (see section “Interaction with other drugs”).
Special information on excipients
The drug Rosuvastatin contains lactose and is therefore contraindicated in patients with lactose intolerance, lactase deficiency, and glucose-galactose malabsorption syndrome.
What is the difference
A comparison of the drugs Atorvastatin and Rosuvastatin revealed that, despite some similarities, they belong to statins of different generations. Rosuvastatin is the latest development, and Atorvastatin precedes it. The advantage of the latest generation is that the dosage of the drug can be reduced, since it is highly effective.
Lovastatin and its analogues
Unlike Atorvastatin, 90% of Rosuvastatin is excreted from the body through the digestive system, and 5% through urine. In addition, if we compare drugs with each other in terms of lowering low-density cholesterol, Atorvastatin is slightly inferior to Rosuvastatin.
The first reduces by a maximum of 54%, and the second by 63%. They also differ in their half-life. If for Atorvastatin this period of time takes from 15 to 30 hours, then for Rosuvastatin it takes 19 hours.
The newest generation drug has a higher bioavailability. This means that it is better absorbed by the body. But the difference between them is not so significant: Atorvastatin – 12% and Rosuvastatin – 20%.
What also makes these drugs different is the nature of their solubility. Thus, Rosuvastatin is a hydrophilic drug, and Atorvastatin is lipophilic. This means that Atorvastatin is fat soluble and Rosuvastatin is water soluble. If you have to make a choice between Atoris and Rosuvastatin, then you must keep in mind that Atoris is a type of Atorvastatin, so the similarities and differences will be approximately the same.
In terms of safety, both drugs under consideration are approximately the same. But, for example, in type 2 diabetes, preference should be given to Rosuvastatin, since it has less effect on carbohydrate metabolism.
If we compare these drugs by cost, then in general the price of Atorvastatin is significantly lower than the cost of Rosuvastatin. In this sense, the dosage of the drug and the number of tablets in the package are important. For example, 90 tablets of 20 mg of Atorvastatin will cost about 800 rubles, while for the same package of Rosuvastatin you will have to pay more than 1000 rubles.
Judging by the effectiveness of these drugs, practice shows that Rosuvastatin has a more pronounced effect compared to Atorvastatin. Their effectiveness is higher, and the potential for side effects is lower than that of first-generation statins, for example, Simvastatin.
Features of Atorvastatin
The drug Atorvastatin belongs to the category of third generation statins. The drug is available in various dosages - 10, 20, 40 and 80 mg. The pharmacy chain offers 2 types of this drug - Russian (Atorvastatin) and Israeli (Atorvastatin-Teva). The active ingredient in Atorvastatin is atorvastatin calcium trihydrate.
The drug reduces the concentration of low-density lipoproteins and simultaneously increases the content of high-density lipoproteins. Also effective for familial and homozygous hypercholesterolemia. The effectiveness of the medicine appears after 2 weeks of regular use. After just 30 days, the maximum effect of the anticholesterol drug is achieved, which persists throughout the entire duration of treatment.
Atorvastatin, like Rosuvastatin, belongs to a new generation of statins
Taking Atorvastatin must be combined with diet. According to the diet, the patient must exclude from the diet foods rich in animal fats, as well as dishes prepared by frying. You can take the tablets regardless of meal time. The dosage is selected individually by the attending physician based on the results of the lipid profile.
The initial dosage is 10 mg; subsequently, if necessary, it can be increased to 80 mg per day. Patients suffering from renal or liver failure take Atorvastatin at this initial dosage all the time. Indications for the use of Atorvastatin are the complex treatment of high levels of total cholesterol and LDL and increased levels of triglycerides.
The following side effects may occur while taking Atorvastatin:
- sleep disturbance;
- headache and dizziness;
- anemia or thrombocytopenia;
- dyspeptic disorders;
- arthritis and myalgia;
- allergy;
- swelling;
- baldness;
- increased sweating;
- sensitivity to light.
Atorvastatin is not prescribed for use in cases of increased activity of liver enzymes, renal failure, during pregnancy and lactation, in case of individual intolerance to the components of the drug, severe liver pathologies. The drug is also contraindicated in patients under 18 years of age.
The cost of the drug in pharmacies depends on the dosage and manufacturer and varies from 130 to 400 rubles. Its analogues are the drugs Atoris, Liprimar, Tulip.
Currently, in developed countries, up to 80–95% of patients with coronary heart disease (CHD) take statins. These figures indicate that cardiologists have no doubt about the advisability of such treatment. Indeed, in recent years, data from several very large studies have been published, which clearly indicate a decrease in mortality (both cardiovascular and general) during therapy with these drugs. In addition, many additional effects of statins have been described and can be used alone. This is, for example, a significant anti-ischemic effect observed with long-term use of these drugs. The anti-inflammatory effects of statins are so great that attempts are being made to treat rheumatoid arthritis with their help. There have been reports of the clinical effectiveness of statins in demyelinating diseases.
However, despite the widespread use of statins, target levels of lipids (primarily atherogenic fractions) are not always achieved. At the same time, a decrease in the incidence of complications of coronary atherosclerosis is associated with the correction of this particular risk factor. Attempts to combine statins with other lipid-lowering drugs are not always successful, as they can lead to the development of severe side effects (as was the case with cerivastatin, which in combination with fibrates led to the development of a large number of cases of rhabdomyolysis). There is evidence of the combined use of statins and nicotinic acid. However, nicotinic acid itself has a very wide range of side effects.
Currently, the use of maximum dosages of statins is actively recommended. However, this makes treatment so expensive that even in developed countries this approach is not widely used. Thus, monthly treatment with atorvastatin (the most popular statin in the United States) at a dose of 80 mg (excluding the cost of monitoring safety parameters) will cost almost $400. In our country, where such a dosage is not registered, taking 8 tablets (10 mg) of atorvastatin per day will cost the patient $400–500 monthly. But even such doses do not always lead to achieving the target level of low-density lipoproteins (LDL).
Comparison of moderate and aggressive lipid-lowering statins
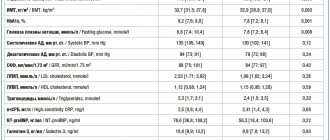
During 2004, the results of 2 interesting studies were published comparing statins - pravastatin, which has a very moderate effect on LDL levels, and atorvastatin, which reduces LDL levels to a much greater extent. The data obtained indicate that the administration of statins, which intensively lower lipid levels, leads to a more pronounced clinical effect.
REVERSAL Study
This study (Reversal of Atherosclerosis with Aggressive Lipid Lowering) compared the effect of 40 mg of pravastatin and 80 mg of atorvastatin per day on the dynamics of atherosclerotic lesions of the coronary bed by changes in the volume of coronary atheroma [1]. Of the 2163 patients screened, 654 patients were randomized and received study drugs. Of these, 502 patients were able to perform intravascular ultrasound of satisfactory quality at baseline and after 18 months of therapy, with 249 patients included in the pravastatin group, 253 in the atorvastatin group. The baseline LDL level in both groups was 150.2 mg/dL (3.89 mmol/L). During treatment with pravastatin, the content of this fraction decreased to 110 mg/dl (2.85 mmol/l), and when using atorvastatin - to 79 mg/dl (2.09 mmol/l). According to this indicator, the differences turned out to be highly significant (p
The studied statins had different effects on atheroma volume. If atherosclerotic lesions progressed during treatment with pravastatin (increase by 2.7%, p = 0.001), then in the atorvastatin group its volume did not change.
PROVE-IT Study – TIMI-22
The Pravastatin or Atorvastatin Evaluation and Infection Therapy – Thrombolysis in Myocardial Infarction 22 (PROVE-IT – TIMI-22) study also compared the effects of atorvastatin (80 mg/day) and pravastatin (40 mg/day) [2].
4162 patients were randomized - men and women over 18 years of age who were hospitalized for acute coronary syndrome (with or without ST-segment elevation) or who had an episode of high-risk unstable angina within 10 days before inclusion in the study. Total cholesterol levels should not exceed 240 mg/dL (6.21 mmol/L) or 200 mg/dL (5.18 mmol/L) if patients had previously received statins. The study did not include patients who had previously received statins at a dose of 80 mg/day, or patients using drugs metabolized by the 3A4 isoenzyme of the cytochrome P450 system.
The pravastatin group included 2063 patients and the atorvastatin group - 2099. The groups were completely comparable in all main clinical characteristics, with the exception of the incidence of peripheral atherosclerosis, which was more often observed among patients receiving pravastatin (p = 0.03). The follow-up period ranged from 18 to 36 months (average 24 months). After 925 endpoints were recorded, the study was stopped.
Endpoints included death from any cause, myocardial infarction, documented unstable angina requiring hospitalization, revascularization (angioplasty or coronary artery bypass grafting) performed more than 30 days after randomization, and stroke.
A significant difference between the groups in LDL levels was noted already from the 30th day of treatment. When using pravastatin, its average concentration was 95 mg/dl (2.46 mmol/l), and in the atorvastatin group it was 62 mg/dl (1.60 mmol/l). In patients previously treated with statins, additional pravastatin did not reduce LDL levels, while atorvastatin reduced their concentration by another 32%. In patients who had not previously taken statins, LDL cholesterol levels decreased by 22% when treated with pravastatin, and by 51% when using atorvastatin. At the same time, the level of high-density lipoproteins (HDL) increased to a greater extent in the pravastatin group (8.1 vs. 6.5%, p
Differences in the degree of reduction in CRP levels also turned out to be highly significant. Although its content significantly decreased in both groups, with pravastatin its level decreased to 2.1 mg/l, and with atorvastatin - to 1.3 mg/l (initial level - 12.3 mg/l in both groups ).
The main result of the study was a 16% (p = 0.005) lower incidence of endpoints in the atorvastatin group. During 2 years of observation, the incidence of adverse outcomes with pravastatin was 26.3%, and only 22.4% with atorvastatin. The difference between the groups began to be noted on day 30 of treatment and persisted throughout the study.
During treatment with atorvastatin, the incidence of unstable angina requiring hospitalization (by 29%, p = 0.02) and the need for revascularization (by 14%, p = 0.04) were significantly lower. Reductions, although not significant, were also observed in the risk of death from any cause (by 28%, p = 0.07) and the combined endpoint of death + myocardial infarction (by 18%, p = 0.06). Stroke was rare in both groups, and there was no significant difference in this endpoint between the groups.
The benefit of atorvastatin was greatest in patients with baseline LDL levels greater than 125 mg/dL (34% risk reduction for the composite endpoint, p = 0.02) and in patients who had not previously received statins.
The importance of the results demonstrating a clear advantage of atorvastatin also lies in the fact that they were obtained in a study conducted by the company that produces pravastatin, whose specialists had reason to assume completely different results. The main hypothesis underlying the protocol was that there would be no difference in the clinical effects of the two statins, despite differences in lipid-lowering effects.
The concept is the lower the better”
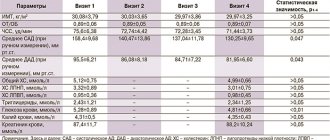
In July 2004, amendments to message 3 of the US National Cholesterol Education Program were published in the journal Circulation [3]. Based on the results of recently published studies, it was decided to significantly tighten the requirements for blood LDL levels (Table 1).
It is proposed to identify 4 risk groups for the development of coronary death over the next 10 years. The high-risk group includes conditions that correspond to the risk of a heart attack or coronary death in more than 20% of cases over the next 10 years. This is a history of coronary artery disease (myocardial infarction, unstable angina, stable angina, revascularization procedures), signs of significant myocardial ischemia. In addition, this risk is caused by the presence of so-called CHD risk equivalents in the patient. This is clinically significant non-coronary atherosclerosis, diabetes mellitus, or a combination of conditions in which the risk exceeds 20%.
In this group of patients, LDL levels should be below 100 mg/dL (2.6 mmol/L). However, there is evidence that this goal may be to reduce LDL concentrations below 70 mg/dL (1.8 mmol/L), especially in patients at very high risk (acute coronary syndrome). If the LDL level in such patients is greater than or equal to 2.6 mmol/l, lipid-lowering therapy is required along with lifestyle changes. However, there is evidence that statin therapy is indicated for such patients even in cases where the initial LDL level is below 2.6 mmol/l. High triglyceride levels or low HDL levels are an indication for additional fibrates or niacin. Moreover, if the concentration of triglycerides is above 2.3 mmol/l, then one of the goals of therapy is to achieve the level of atherogenic fractions of cholesterol (cholesterol of all fractions except HDL) below 3.4 mmol/l (0.8 mmol/l above the target LDL level ).
The next gradation – high intermediate risk – involves a combination of 2 or more risk factors, in which the incidence of myocardial infarction or coronary death over the next 10 years is 10–20%. This risk is caused by a combination of smoking and an increase in blood pressure over 140/90 mm Hg. Art., low HDL level (
The risk based on these factors is calculated using special calculator programs (on the Internet, such a calculator is located at www.nhlbi.gov/guidelines/cholesterol).
Intermediate risk is a combination of 2 or more risk factors for which the 10-year risk calculated using a calculator does not exceed 10%.
When lipid-lowering drugs are used in high- and intermediate-risk groups, LDL levels should be reduced by at least 30–40%.
It is also important that all patients actively adjust their lifestyle (increase in physical activity); carried out therapy aimed at reducing excess body weight and treating metabolic syndrome, eliminating hypertriglyceridemia and increasing HDL levels if they decreased.
In the low-risk group (0–1 risk factor), the risk of CAD should not be calculated.
European recommendations for the prevention of cardiovascular diseases are based not on calculating the risk of complications of coronary atherosclerosis, but on taking into account the total risk of cardiovascular diseases, including stroke.
The place of the anti-inflammatory effect in the antiatherogenic action of statins
The large number of effects of statins that are not associated with a decrease in the level of atherogenic lipoproteins allows us to seriously discuss the question of what is more important - their effect on cholesterol synthesis or other consequences of blockade of mevalonic acid metabolism.
The anti-inflammatory effect of statins has received the most attention. The participation of inflammatory reactions in atherogenesis is now considered proven. It has been established that the prognosis for atherosclerotic lesions of various locations can be significantly clarified by measuring the level of inflammatory markers in the systemic circulation. Most often, this is done by determining the level of CRP using a highly sensitive technique. Based on data from large epidemiological studies, it is proposed to divide the CRP level into 3 ranges: low - less than 1.0 mg/l, medium - 1.0-3.0 mg/l and high - more than 3.0 mg/l. A CRP level above 10 mg/L indicates the presence of causes for a systemic inflammatory response, such as infection, trauma, etc. To assess the risk of atherosclerosis, this test should be repeated 2 weeks after the relief of these conditions.
CRP is a non-glycosylated protein, the main regulator of whose synthesis is interleukin 6. The stimulus that provokes the synthesis of CRP can be oxidized lipoproteins. Until recently, it was believed that CRP is synthesized by hepatocytes, but recently it has been discovered that it can also be produced in atherosclerotic plaques, kidneys, nervous tissue and alveolar macrophages. Recently, information has emerged that CRP is not only a marker of the inflammatory process in atherosclerosis, but is itself an active factor in atherothrombosis.
The effect of CRP on endothelial cells leads to a decrease in the activity of endothelial NO synthetase, a decrease in the synthesis of prostacyclin, an increase in the production of type 1 plasminogen activator inhibitor, endothelin-1, interleukins 6 and 8, and some cell adhesion molecules that provoke the migration of macrophages into the subendothelial layer. The proatherogenic effect of CRP on smooth muscle cells is manifested by the acceleration of their proliferation, increased expression of angiotensin type 1 receptors and an increase in the level of inducible NO synthetase. The latter synthesizes nitric oxide, which is spent mainly on the formation of a very aggressive radical - peroxynitrite. Macrophages under the influence of CRP more actively produce free radicals, cytokines (interleukins 1 and 6, tumor necrosis factor), tissue factor, etc. [4]. All this confirms the direct participation of CRP in the processes of atherogenesis. Therefore, reducing its level can be considered as one of the main goals of pathogenetic treatment of atherothrombosis.
To date, several studies have been published comparing the effects of different statin regimens on CRP levels. A randomized, placebo-controlled trial of 110 patients compared the effects of treatment with atorvastatin (10–80 mg, target LDL level less than 2.1 mmol/L) and lovastatin (5–10 mg, target LDL level less than 3.4 mmol/L). l). A significant decrease in CRP levels (from 2.6 to 1.7 mg/l, p = 0.002) was achieved only with intensive lipid-lowering therapy with atorvastatin. In other words, there is a parallel between the effects of statins on lipids and inflammatory markers.
Rosuvastatin is the most effective statin
Rosuvastatin (Crestor) is a completely synthetic drug of the statin group, approved for clinical use in 2003. Due to its obvious advantages, the demand for rosuvastatin turned out to be very high. This is evidenced by the fact that since registration it has already been prescribed to more than 2.5 million patients, despite the presence of 5 other statins on the pharmaceutical market.
The pharmacokinetic characteristics of rosuvastatin and other currently used statins are presented in table. 2. After a single dose of rosuvastatin, its maximum concentration in the blood is reached after approximately 5 hours. The drug has the longest half-life - 19 hours. This may be due to the phenomenon of enterohepatic recirculation demonstrated experimentally. Approximately 88% of rosuvastatin is reversibly bound to plasma proteins, mainly albumin.
The pharmacokinetic characteristics of rosuvastatin are not affected by age, gender, time of drug administration or concomitant food intake, as well as the presence of moderate liver failure.
The rosuvastatin molecule is hydrophilic, which makes it more selective towards hepatocytes than other tissues. Another consequence of the hydrophilicity of this drug is its significantly less effect on cholesterol synthesis in skeletal muscle myocytes. In studies conducted on cultured skeletal muscle cells, rosuvastatin and another hydrophilic statin, pravastatin, reduced the activity of cholesterol synthesis to a much lesser extent (50-1000 times) than the lipophilic atorvastatin, simvastatin and cerivastatin. This fact allows us to consider rosuvastatin potentially safer compared to other drugs from the statin group.
It has been shown that 90% of the drug is excreted in feces, and 10% in urine. Only 10% of rosuvastatin is metabolized; its main metabolite, N-desmethyl rosuvastatin, is approximately 2 times less active than the main substance. Unlike most statins (in particular, atorvastatin and simvastatin), rosuvastatin interacts minimally with enzymes of the cytochrome P450 system, and therefore the likelihood of its interaction with many widely prescribed drugs (antihistamines, antifungals, antiulcers, a number of antibiotics, antidepressants, cardiac glycosides, etc.) is minimal. In particular, the drug does not have an inhibitory effect on isoenzymes 1A2, 2C19, 2D6, 2E1 and 3A4. Only for the 2C9 isoenzyme, a decrease in its activity by 10% was noted. When rosuvastatin was co-administered with fluconazole (a potent inhibitor of the 2C9 isoenzyme), only a slight increase in statin concentrations was observed.
The clinical effectiveness of rosuvastatin is being actively studied in a series of studies, the program of which is called GALAXY (45 thousand participants). This program examines the effects of rosuvastatin on lipids, inflammatory markers, the dynamics of the atherosclerotic process and so-called fixed (or “hard”) endpoints. The program covers most of the conditions for which statins are currently used.
However, the greatest interest is in the AURORA study, which examined the effect of the drug in patients on hemodialysis; the CORONA trial, which included patients with heart failure, and the JUPITER trial, which focused on primary prevention in patients with normal LDL levels and elevated CRP levels. All these studies are ongoing and their results will resolve a number of unexplored issues.
Among those completed, the MERCURY I (Measuring Effective Reductions in Cholesterol Using Rosuvastatin therapy) study was of great importance for assessing the effectiveness of rosuvastatin. This open-label, randomized trial included 3161 patients with hyperlipidemia [5]. Its purpose was to compare the effectiveness of rosuvastatin, atorvastatin and other statins in terms of reducing LDL concentrations. The main criterion for assessing effectiveness was achieving the target LDL level. The study lasted 16 weeks.
The result of the study was the demonstration of a significant advantage of rosuvastatin over other statins in terms of their effect on lipids. The target LDL level while taking rosuvastatin at a dose of 10 mg/day was achieved in 86% of patients compared to 80% when using a similar dose of atorvastatin (p
Currently, rosuvastatin is recommended for use in dosages of 10–40 mg per day. At the same time, LDL levels are reduced by 52–63%, i.e. to a much greater extent than when using other statins in similar doses. In addition, treatment with rosuvastatin significantly increased the concentration of HDL by 14% and decreased the level of triglycerides by 28%.
Reducing the level of atherogenic fractions of cholesterol (primarily LDL cholesterol) is not the only goal of lipid-lowering therapy. It should also be aimed at correcting the content of HDL, a potentially antiatherogenic fraction of lipoproteins, the level of which is reduced in many patients.
At the last European Congress of Cardiology (2004), the results of the largest population-based study INTER-HEART were presented. It showed that 9 potentially modifiable factors determine the risk of myocardial infarction by 90%, with the most significant of them being the ApoB/ApoA1 ratio. In second place in importance is smoking, followed by diabetes mellitus, arterial hypertension, abdominal obesity, psychosocial factors, the amount of fruits and vegetables consumed, physical inactivity and alcohol consumption [6].
The fact that the most significant was the ratio of ApoB, the only protein included in LDL, to ApoA1, the HDL protein, once again confirms the special importance of maintaining this fraction of lipoproteins at an optimal level. The main competitor of rosuvastatin, atorvastatin, has a very slight effect on HDL concentrations. Moreover, as the dose of atorvastatin increases, the extent of its effect on HDL decreases.
Rosuvastatin behaves in the opposite way. The STELLAR study [7] showed that differences between it and atorvastatin in the effect on HDL appeared already when using these drugs at a dose of 10 mg, and when taking statins at a dose of 20 mg, the difference became highly significant. When using rosuvastatin at a dose of 10 mg, the level of ApoB decreased by 38%, and the concentration of ApoA1 increased by 5%. As a result, the ApoB/ApoA1 ratio decreased by 40%.
The pronounced hypotriglyceridemic effect of rosuvastatin can be considered proven in patients with initial triglyceride levels in the range from 3.4 to 9 mmol/l [8]. A randomized, placebo-controlled study that included 156 such patients showed that a 6-week course of rosuvastatin therapy leads to a decrease in triglyceride levels by 37–40%, and cholesterol of all atherogenic fractions by 42–51%. In parallel, HDL levels increase by 6–18%. This feature of the action of rosuvastatin allows us to expect that its widespread introduction into clinical practice will significantly reduce the need for the combined prescription of statins and fibrates.
Safety of rosuvastatin
The problem of treatment safety is the most important aspect of the use of any medicine. It becomes especially important when introducing into clinical practice a drug intended for a very large number of patients. Attention to the safe use of statins has increased sharply after the withdrawal of cerivastatin from the market, the uncontrolled use of which led to a large number of fatal complications.
The emergence of a new drug in this class, rosuvastatin, opened a discussion about the advisability of registering a new drug in conditions where a significant number of statins already exist and are actively used. That is why rosuvastatin has undergone very careful study from a safety point of view. Information for this drug was analyzed on twice the number of patients required for registration of other statins. One of the consequences of this test was some delay in the release of the drug to the market. On the other hand, its results allow us to confidently assert that, subject to dosages and rules for prescribing rosuvastatin, its use is at least as safe as other statins.
However, some precautions common to all statins must be taken when using rosuvastatin. Thus, in some patients, while using high doses of rosuvastatin, proteinuria may be observed, associated with the development of a kind of tubulopathy, which is also observed in 0.2–0.6% of cases when using other statins. Therefore, the drug should not be prescribed in a dose of 40 mg to patients with creatinine clearance less than 60 ml/min and in any dosage with creatinine clearance below 30 ml/min. In any case, when prescribing 40 mg of rosuvastatin, it is necessary to monitor the level of proteinuria.
Like other statins, rosuvastatin should be used cautiously in patients who abuse alcohol and have liver disease. All patients need to determine the level of liver transaminases initially and after 3 months of therapy. An increase in their level by more than 3 times is a reason to reduce the dose or discontinue the drug.
Pharmacokinetic studies have shown that patients belonging to the Mongoloid race are more sensitive to the effects of statins. Therefore, when prescribing rosuvastatin to such patients, slightly lower dosages should be used and the drug should not be used at a dose of 40 mg.
The most serious, although extremely rare, side effect of any statin is the development of rhabdomyolysis. Before starting the use of rosuvastatin, it is necessary to measure the initial level of creatine phosphokinase. If it increases more than 5 times after 5-7 days, the analysis must be repeated - if the increase persists, rosuvastatin therapy is not started. It was found that myopathy and rhabdomyolysis are more common in patients with kidney damage and hypothyroidism; in the presence of congenital muscle diseases in relatives; in patients who had signs of muscle tissue damage while taking other statins and fibrates; in persons who abuse alcohol; in cases where the drug is used in combination with fibrates. Such patients should not be prescribed rosuvastatin at a dose of 40 mg. The presence of myopathy is a contraindication to the use of the drug in all dosages.
Rosuvastatin is also contraindicated in women during pregnancy and lactation. It should not be prescribed to patients with preserved reproductive function if they do not use contraception.
In general, the safety profile of rosuvastatin does not differ from that of other statins. To date, its safety has been studied in more than 15 thousand patients, 1500 patients have taken the drug for more than 2 years. More than 45 thousand people are currently participating in controlled studies of rosuvastatin.
Thus, the feasibility of the widest use of statins in clinical practice does not raise any doubts today. In our country, as well as throughout the world, there are practically no doctors left who are unfamiliar with the therapeutic possibilities provided by drugs of this class. Unfortunately, statins are not always used correctly, in adequate dosages, which, of course, significantly reduces the effectiveness of their use as a means of preventing severe complications of atherosclerosis. A new representative of this class, rosuvastatin (Crestor), now available in Russia, surpasses all other statins in its ability to reduce the level of proatherogenic lipoproteins. Its most important advantages are also its beneficial effect on antiatherogenic fractions of lipoproteins (primarily HDL) and a powerful hypotriglyceridemic effect. This reduces the need for combined administration of statins and fibrates, which significantly increases the safety of lipid-lowering therapy. In addition, the effectiveness of the drug in most patients at an initial dose of 10 mg reduces the need for titration compared to other statins, increases adherence to treatment, and reduces the cost of therapy. Some features of the pharmacokinetics and pharmacodynamics of rosuvastatin allow us to expect better tolerability of this drug compared to other statins.
Features of Rosuvastatin
Rosuvastatin is a lipid-lowering agent and is prescribed to normalize the level of triglycerides and phospholipids and lipoproteins in the blood. The main active ingredient is rosuvastatin. It is prescribed for the treatment and prevention of hypercholesterolemia, atherosclerosis and its complications, and vein thrombosis.
Rosuvastatin reduces high concentrations of low-density cholesterol, total cholesterol, triglycerides, and apoliprotein. At the same time, it helps to increase the level of high-density lipoproteins. While taking this drug, the atherogenicity index decreases. This effect indicates normalization of the lipid profile.
Indications for prescribing this drug are as follows:
- hereditary tendency to increase cholesterol;
- initial detection of high cholesterol levels in the blood;
- increased levels of triglycerides in the blood;
- prevention of complications of atherosclerosis, namely angina pectoris, hypertension, myocardial infarction and stroke in patients over 50 years of age.
Since Rosuvastatin is not metabolized in the liver, its effect on this organ is minimal. After 90% of the drug is excreted through the intestines with feces, the rest through the kidneys with urine. The drug is not prescribed to persons under 18 years of age, patients with lactose intolerance, women during pregnancy and breastfeeding.
It is contraindicated in patients at the acute stage of liver disease, patients with myopathy, and those with individual intolerance to the components of the drug. The combination of Rosuvastatin with Cyclosporine is unacceptable. During treatment with this statin, doctors recommend following an anti-cholesterol diet. When prescribing a drug in a dosage of more than 40 mg, the attending physician takes into account the absolute and relative indications for this.
Rosuvastatin lowers cholesterol levels better than all other similar drugs
Both drugs - Atorvastatin and Rosuvastatin - have good patient reviews. Since these medications have similar effects, they are interchangeable. But the choice of a particular one should be determined by the doctor in accordance with the diagnosis and individual characteristics.
Rosuvastatin is the optimal drug for the treatment and prevention of atherosclerosis
Correction of lipid metabolism disorders occupies a leading position in this area [1]. The first-line drugs in the treatment of dyslipidemia in patients with risk factors for CVD associated with atherosclerosis are, of course, statins (3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors - HMG-CoA reductase). The main goal of using statins in all patients is to slow the progression of atherosclerosis, which, in turn, reduces the risk of complications and improves the prognosis. Currently, millions of patients receive drugs of this class for the purpose of secondary and primary prevention [2]. Over the past 20 years, statins have repeatedly demonstrated their effectiveness in various large studies. Thus, in randomized clinical trials 4S, HPS, ASCOT-LLA, LIPID, it was shown that long-term use of these drugs reduces the number of recurrent complications of coronary heart disease (CHD), myocardial infarction (MI), unstable angina and deaths by 25–40% , ischemic strokes – by 25–30% [3–6]. At the same time, there is a decrease in the levels of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) by 25–30% and 30–35%, respectively. However, despite significant advances in the treatment of CVD, the risk of complications remains high. Currently, six statins are publicly available: lovastatin, pravastatin, simvastatin, fluvastatin, atorvastatin and rosuvastatin. Among the listed drugs, rosuvastatin occupies a special place, which is due to its undeniable advantages in terms of pharmacological and clinical properties compared to other statins. Rosuvastatin is a synthetic statin that interacts to a greater extent with HMG-CoA reductase, which is associated with a higher affinity for the active center of this enzyme [7]. This drug also has the longest half-life of all statins and is the only statin that is minimally metabolized by the cytochrome P450 (CYP 450) enzyme system without significant involvement of the 3A4 isoenzyme. As a result, clinically significant drug interactions between rosuvastatin and other drugs that inhibit CYP 450 enzymes practically do not occur [8]. This property of rosuvastatin facilitates its use as part of complex therapy, for example, with angiotensin-converting enzyme inhibitors, nitrates, calcium antagonists, diuretics, beta-blockers. As for the safety and tolerability of rosuvastatin, it is comparable to those for other statins – atorvastatin, simvastatin and pravastatin – which was shown in more than 12 thousand patients [7]. Clinically significant elevations in liver enzymes and creatine kinase occurred infrequently, occurring in no more than 0.2% of cases, and myopathy occurred in approximately 0.03% of cases among patients taking rosuvastatin at doses up to 40 mg. When rosuvastatin was used at a dose of 5–40 mg, no drug-related deaths or rhabdomyolysis were reported [9]. The rosuvastatin molecule is more hydrophilic than other statin molecules, is highly selective for hepatocyte membranes and has a much more pronounced inhibitory effect on the synthesis of LDL cholesterol than other statins. The drug is quickly absorbed from the intestine, regardless of the accompanying meal and time of day. The peak concentration of rosuvastatin in plasma is reached 3–5 hours after administration, the half-life is 18–20 hours. Up to 90% of the drug taken is excreted unchanged through the gastrointestinal tract, about 10% is excreted in the urine. Enterohepatic recirculation provides a relatively long half-life of rosuvastatin, however, the initial decrease in the functional activity of the liver does not affect the pharmacokinetics of the drug. One of the main features of rosuvastatin should be considered its high lipid-lowering effectiveness already at the initial daily dose (10 mg/day), which increases with increasing dose to the maximum. It has been established that at a dose of 10 mg/day. rosuvastatin reduces the level of LDL cholesterol by 42–52% from the initial level and by 63% when prescribing the maximum permitted dose (40 mg/day). The main goal of taking statins is to achieve the target level of LDL cholesterol: less than 2.5 mmol/l in patients with a high risk of coronary artery disease and less than 1.8 mmol/l in patients with a very high risk, and it is rosuvastatin that is most effective in this regard in the majority patients compared to other statins [10]. This conclusion has been reached in a number of clinical studies. Since rosuvastatin appeared on the market later than other statins, most clinical studies used atorvastatin as a control, more effective drug. A randomized, double-blind, multicenter study compared the effectiveness of atorvastatin and rosuvastatin in lowering LDL cholesterol levels in patients with hypercholesterolemia and high risk of coronary artery disease. It turned out that at the 12th week of therapy, rosuvastatin at a dose of 10 mg significantly reduced LDL cholesterol levels to a greater extent than atorvastatin at a dose of 10 mg (47% and 35%, respectively) [11]. Another study, which involved more than 3 thousand patients with coronary artery disease, atherosclerosis and type 2 diabetes mellitus, assessed the effectiveness of switching to low doses of rosuvastatin from therapy with atorvastatin, simvastatin and pravastatin. The effectiveness of therapy was assessed by the number of patients who reached an LDL cholesterol level of <3 mmol/l at week 16. It was shown that switching to rosuvastatin at a dose of 10 mg allowed patients to achieve target LDL cholesterol levels significantly faster compared to those patients who continued treatment with atorvastatin at a dose of 10 mg (86% vs. 80%), simvastatin at a dose of 20 mg (86 % vs. 72%) and pravastatin at a dose of 40 mg (88% vs. 66%) [12]. In the randomized, open-label, multicenter SOLAR trial, which was conducted at 145 centers in the United States, men and women at high risk for CAD were prescribed starting doses of rosuvastatin (10 mg), atorvastatin (10 mg), or simvastatin (20 mg) for 6 weeks. For those patients who did not achieve LDL cholesterol levels <100 mg/dL by week 6, the dose was doubled and observed for the next 6 weeks. A total of 1632 patients were randomized into three treatment groups. After 12 weeks 76% of patients treated with rosuvastatin achieved target LDL cholesterol levels, compared with 58% and 53% of patients treated with atorvastatin and simvastatin. Side effects occurred with equal frequency in all groups, and only 3% of all patients discontinued treatment due to side effects [13]. As for the effect of rosuvastatin therapy on the level of high-density lipoprotein cholesterol (HDL-C), which is an independent marker of cardiovascular risk, the beneficial effect of this drug has been proven. Thus, in a randomized, open-label comparative study in patients with metabolic syndrome, the effect of therapy with rosuvastatin and atorvastatin on plasma lipid levels in individuals with hypercholesterolemia was assessed. As a result, during treatment with rosuvastatin, an increase in HDL cholesterol levels by 8–11% was observed [14]. The effectiveness of rosuvastatin was also studied in patients with severe heterozygous familial hypercholesterolemia. The study compared the effectiveness of rosuvastatin and atorvastatin in lowering LDL cholesterol levels: 623 patients were randomized into 2 groups to receive treatment at a dose of 20 mg atorvastatin (187 people) or rosuvastatin (436 people) and forced dose titration at 6-week intervals to 80 mg/day. days At 18 weeks During therapy with rosuvastatin, there was a more significant decrease in LDL cholesterol levels (–57.9% vs. –50.4%) and an increase in HDL cholesterol levels (12.4% vs. 2.9%) than with atorvastatin therapy [15]. Also, the use of rosuvastatin turned out to be effective in terms of primary prevention of cardiovascular complications. Thus, in the randomized, double-blind, placebo-controlled trial JUPITER to study primary prevention in individuals with normal and low levels of LDL cholesterol and increased cardiovascular risk due to an increase in the concentration of high-sensitivity C-reactive protein (hs-CRP), favorable results were obtained [16] . The prerequisite for this study was the data that about half of all cardiovascular complications develop in practically healthy individuals with normal or reduced levels of LDL cholesterol. In these individuals, new prognostic markers need to be established to study the risk of developing CVD. It was previously established that hs-CRP is a marker of cardiovascular pathology in individuals with normal or low levels of LDL cholesterol. The indicator is recognized as a marker of inflammation and is associated with an increased risk of developing atherosclerosis. Therefore, in addition to improving the identification of people at risk, the goal was to study the possibilities of lipid-lowering therapy as one of the methods of primary prevention of cardiovascular complications. The study included 17,802 patients from 26 countries with LDL cholesterol <3.36 mmol/l and hs-CRP levels >2 mg/dl, who were randomized into 2 groups: in group 1, patients took rosuvastatin at a dose of 20 mg /day, in the 2nd - placebo. Most patients were overweight or obese. In March 2008, a decision was made to terminate the JUPITER clinical trial early because there was clear evidence of a reduction in CVD and mortality among patients treated with rosuvastatin compared with those treated with placebo (mean duration of approximately 2 years). The final data from the JUPITER study [17] indicate that in the rosuvastatin group there was a significant reduction in the risk of developing the primary end point (MI, stroke, revascularization, hospitalization with unstable angina, death from cardiovascular causes) by 44%; MI – by 54%; stroke – by 48%; death from cardiovascular causes – by 47% and death from all causes – by 20%. In summary, this study of the effectiveness of primary prevention in individuals without hyperlipidemia but with elevated hs-CRP levels demonstrated the significant effectiveness of rosuvastatin in reducing the incidence of major cardiovascular events. The randomized, double-blind, placebo-controlled METEOR trial determined whether treatment with rosuvastatin slows progression and/or reverses atherosclerosis (based on intima-media thickness (IMT)) in low-risk individuals with subclinical atherosclerosis. The study included 984 patients (men and women aged 45–70 years) without coronary artery disease, with moderate hypercholesterolemia (average LDL cholesterol 154 mg/dl), who had initial IMT values in the range of 1.2–3.5 mm, whose risk factor was age or a 10-year fatal risk of less than 10% according to the Framingham scale. Patients received rosuvastatin 40 mg or placebo for 2 years. The results showed that in the rosuvastatin group, the average level of LDL cholesterol decreased by 49%. Changes in maximum IMT were –0.0014 mm/year in the rosuvastatin group and +0.0131 mm/year in the placebo group. Overall, in middle-aged people with <10% risk and subclinical atherosclerosis, treatment with rosuvastatin compared with placebo for 2 years significantly reduced the progression of atherosclerosis [18]. Non-invasive magnetic resonance imaging (MRI) imaging was used to study the effect of rosuvastatin on carotid atherosclerosis, atherosclerotic plaque morphology and composition in the randomized, double-blind ORION trial. 43 patients were divided into 2 groups: the first took rosuvastatin at a low dose of 5 mg, the second - at a high dose of 40/80 mg. MRI was performed at baseline and after 24 months. therapy. After 2 years of follow-up, it was shown that in patients with moderate hypercholesterolemia, rosuvastatin therapy led to a relative reduction in the lipid-rich necrotic plaque core without a significant change in their number [19]. Thus, it can be assumed that long-term treatment with rosuvastatin leads to stabilization of atherosclerotic plaques. Rosuvastatin also demonstrated its positive effect in the case of coronary atherosclerosis, which was revealed in the prospective open study ASTEROID. After 24 months therapy, 349 patients underwent intravascular ultrasound (IVUS). Against the background of intensive therapy with rosuvastatin at a dose of 40 mg/day. managed to achieve not only a decrease in blood lipid levels - LDL level by 60.8 mg/dl and an increase in HDL by 14.7%, but also caused a significant regression of atherosclerotic lesions of the coronary arteries, which was assessed by all studied IVUS indicators [20]. In the same study, coronary angiography was used and the degree of narrowing and the minimum lumen of the coronary arteries and their main branches at different levels were determined as a percentage, with the initial stenosis being >25%. For each patient, mean lesion scores were calculated at the beginning and end of the study. In total, 613 stenoses were identified in 292 patients. Therapy with rosuvastatin caused regression of atherosclerosis in the form of a decrease in the relative diameter of the stenosis and an increase in the diameter of the vessel lumen according to coronary angiography in patients with coronary artery disease [21]. Also, the effectiveness of rosuvastatin was studied in the large GALAXY clinical program, which included 18 multicenter randomized controlled trials. This program examined the relationship between optimal lipid control, atherosclerosis, and cardiovascular morbidity and mortality. Research can be divided into three broad categories: 1) studying the effect of rosuvastatin on lipids and inflammatory markers; 2) study of the effect of rosuvastatin on atherosclerotic lesions of the coronary and carotid arteries; 3) study of the effect of rosuvastatin on the risk of developing cardiovascular complications, cardiovascular and overall mortality. As a result, the benefits of rosuvastatin were shown both in the normalization of lipid metabolism, markers of inflammation, and in the reverse development of atherosclerosis in the coronary and carotid arteries [2]. Thus, in combination with data from the JUPITER study, rosuvastatin is currently the most promising drug in the prevention of the development and progression of atherosclerosis. More than 20 years of experience with statins has shown that these drugs are well tolerated by the vast majority of patients. A large number of multicenter clinical studies were conducted, in which a total of more than 100 thousand patients participated, the duration of observation reached 5 years or more. There were no indications of severe side effects directly related to these drugs. Concerns about the possible influence of statins on the central nervous system, sleep, and a negative impact on human mental activity were not confirmed. Moreover, the results of recent studies have shown that statins, when taken long-term, help prevent the development of dementia and Alzheimer's syndrome. Among all statins, the most promising is the use of rosuvastatin. The generic rosuvastatin Mertenil (Gedeon Richter, Hungary) has been registered and successfully used in Russia. The appearance of Mertenil gives reason to assume that this effective remedy will become available to a wide range of patients, because the use of the original drug is not always possible from an economic point of view. Mertenil is in no way inferior to the original drug in terms of pharmacological properties, dosage form, potency, route of administration and quality. Mertenil is also the only rosuvastatin in Russia with a full range of dosages (5, 10, 20 and 40 mg), which is very convenient for dose selection and use in various clinical situations. Convenient packaging, designed for 1 month of use (30 tablets), increases the patient's adherence to treatment. An important role is played by affordability for long-term use of the drug. Thus, Mertenil can be recommended to patients for primary and secondary prevention of CVD, including those without clinical signs of coronary artery disease. Literature 1. Diagnosis and correction of lipid metabolism disorders for the purpose of prevention and treatment of atherosclerosis. Russian recommendations. // Cardiovascular therapy and prevention. – 2007. – No. 6 (Appendix 3). 2. Karpov Yu.A. Lipid-lowering therapy as an important component in the treatment and prevention of cardiovascular diseases. // RMJ – 2011. – T. 19, No. 7. – P. 450–456. 3. Scandinavian Simvastatin Survival Study Group: Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study. // Lancet 1994: 344: 1383–1389. 4. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20536 high–risk individuals: a randomized placebo–controlled trial. // Lancet 2002; 360:7–22. 5. Sever PS, Dahlof B, Poulter NR et al. for the ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower–than–average cholesterol concentrations, in the Anglo–Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT–LLA): a multicentre randomized controlled trial. // Lancet 2003; 361:1149–1158. 6. The Long–term Intervention with Pravastatin in Ischemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. // N Engl J Med. 1998;339:1349–1357. 7. P. Rubba, G. Marotta, M. Gentile. Efficacy and safety of rosuvastatin in the management of dyslipidemia. // Vasc Health Risk Manag. 2009; 5:343–352. 8. Mckennyy JM Efficacy and Safety of Rosuvastatin in Treatment of Dyslipidemia. // Am J Health Syst Pharm. 2005; 62: 1033–1047. 9. Shepherd J., Hunninghake DB, Stein Ea et al. Safety of rosuvastatin. // Am J Cardiol. 2004; 94: 882–888. 10. Larosa JC Low - Density Lipoprotein Cholesterol Reduction: The End is More Important than the Means. // Am J Cardiol. 2007; 100: 240–242. 11. Schwartz GG, BOLOGNESE MA, Tremblay Bp et al. Efficacy and Safety of Rosuvastatin and Atorvastatin in Patients with Hypercholesterolemia and a High Risk of Coronary Heart Disease: a Randomized, Controlled Trit. // Am Heart J. 2004; 148–154. 12. Schuster H., Barter Pj, Stender S. et al. Effective Reductions in Cholesterol USING ROSUVASTATIN THEERAPY ITYAPY GROUP EFFects of Switching Statins On Achievement of Lipid Goals: Measurging Effective Readuction Cholesterol Using Rosuvastatin Therapy (Mercury I) Study. // Am Heart J. 2004; 147: 705–713. 13. Insull W., Jr, Ghali JK, Hassman Dr et al. Solar Study Group Achieving Low - Density Lipoproprotein Cholesterol Goals in High -Risk Patients in Managed Care: Comparison of Rosuvastatin, Atorvastatin, and Simvastatin in the Soleg . Mayo Clin Proc. 2007; 82: 543–550. 14. Deedwania PC, Hunninghake DB, Bays He, Jones PH, Cain VA, Blassetto JW, Stellar Study Groups of Rosuvastatin, Atorvastatin, Simvastatin, and Pravastatin on Abherogenic D Yslipidemia in Patients with Characteristics of the Metabolic Syndrome. // Am J Cardiol. 2005; 95: 360–366. // Am J Cardiol. 2003; 92: 1287–1293. 16. Ridker pm, fonseca fa, Genest J. et al. Jupiter Trial Study Group Baseline Characteristics of Participants in The Jupiter Trit, a Randomized Placebo - Controlled Primary Prevention Thatin therapy Among Individuals ITH Low Low - Density Lipoprotein Cholesterol and Elevated High -Sensitivity C - Reactive Protein. // Am J Cardiol. 2007; 100: 1659–1664. 17. Ridker PM, Danielson E., Fonseca Fa et al. The Jupiter Study Group Rosuvastatin to Prevent Vascular Events in Men and With Elevated C - Raactive Protein. // N Engl J Med. 2008; 359 (2): 2195–2207. 18. Crouse Jr, Raichlen Js, Riley Wa et al. Meteor Study Group Effect of Rosuvastatin on ProgReSSion of CAROTIM - Media Thickness in Low - Risk Individuals with Subclinical atherosclerosis: The Meteor Trial. // JAMA. 2007; 297: 1344–1353. 19. Underhill HR, Yuan C., Zhao XQ et al. Effect of Rosuvastatin Therapy on Carotid Plaque Morphology and Composition in Moderately Hypercholemic Patients: A High -ReSolution Magnance Imaging Trigal. // Am Heart J. 2008; 155: 584.e1 - E8. 20. Nissen Se, Nicholls SJ, Sipahi I. et al. Asteroid Investigators. // JAMA. 2006; 295: 1556–1565. 21. Ballantyne CM, Raichlen JS, Nicholls SJ et al. Asteroid Investigators Effect of Rosuvastatin Therapy on Coronary Artry Stenoses Assessed by Quantitive Coronary Angiography: A Study to Effect of RosuvaVaVaVASTATATI OnTravascular Ultrasound - Derved Coronary Atheroma Burden. // Circulation. 2008; 117: 2458–2466.
Reviews
Oksana, 46 years old, Pyatigorsk: I took Atorvastatin to lower cholesterol. It reduces well, but I experienced side effects myself. After 3 months of regular use, almost all lipid profile indicators, except triglycerides, approached normal. Based on the results of the analysis, the doctor supplemented the treatment with Dibikor, and triglycerides also decreased. I was afraid for my liver, but in vain. Everything is fine there.
Ekaterina, 35 years old, Moscow: I can’t say that Atorvastatin is significantly different in effectiveness from previous drugs. My father was prescribed 60 mg per day; he has high cholesterol. He says that he does not notice any significant changes in his condition after the course of treatment. Cholesterol remains what it was. Apparently this is due to hereditary predisposition.
Kirill, 60 years old, Samara: With a diagnosis of coronary heart disease, the doctor prescribed statins to prevent complications. The choice fell on the drug Rosuvastatin. I don’t feel its influence on myself, but according to tests, cholesterol corresponds to the norm for a healthy person. Before starting to take Rosuvastatin, it was increased several times. I can confidently recommend this drug to others to reduce high cholesterol.