Introduction
A decrease in ovarian reserve (OR) is a pressing problem not only in women of late reproductive age, but also in patients with premature ovarian failure (POF) [1]. Increasingly, women are postponing the implementation of reproductive function, which raises the problem of reduced fertility in late reproductive age. The ovarian reserve is established in utero, and throughout life it gradually decreases. When the follicular reserve is depleted, menopause occurs. Currently, the level of anti-Müllerian hormone (AMH), the number of antral follicles according to transvaginal ultrasound (US) in the early follicular phase, and the level of follicle-stimulating hormone (FSH) measured on days 2-4 of the menstrual cycle are used as diagnostic criteria for SOP. SOR is diagnosed when an AMH level is ≤1 ng/ml and a decrease in the number of antral follicles [2]. This condition is accompanied by an increase in FSH levels >25 U/l, which in patients under 40 years of age is defined as premature ovarian failure, at the age of 40-45 years - early menopause, after 45 years - timely menopause. The estimated incidence of POF is 1–2% of the general population, with early menopause occurring in 5–10% of women [3].
Causes of POI: idiopathic, iatrogenic (bilateral oophorectomy or chemotherapy), severe infectious processes, autoimmune diseases, genetic diseases (the most common are X chromosome defects) [2]. Risk factors include: family history, early menarche, no history of childbirth, smoking, underweight [4].
POI and early menopause increase the risk of mortality from all causes—cardiovascular disease, type 2 diabetes, depression, osteoporosis, and fractures [5, 6]. The association between early menopause and dementia has not been confirmed, but there is evidence that POI is associated with a higher risk of cognitive decline later in life [7, 8]. Currently, therapy with estrogens and gestagens is considered as the optimal way to prevent long-term consequences of estrogen deficiency, and treatment should begin as early as possible and continue at least until the middle age of menopause (50-52 years), unless it is contraindicated (for example, in women with hormone-dependent cancer). Given the young age of patients, higher doses of drugs may be required to relieve symptoms or protect bone tissue [4].
The functioning of the female reproductive system depends on the proper development and regulation of the hypothalamic-pituitary-ovarian axis (HPA), its main integrating element is gonadotropin-releasing hormone (GnRH). It was discovered in the early 70s of the last century, and in 2000 a group of authors [9] isolated and determined the chemical structure of the hypothalamic neuropeptide in birds, which inhibits the release of GnRH and gonadotropins, and named it gonadoinhibin. After the discovery of gonadoinhibin and its inhibitory effect on the hypothalamic-pituitary-gonadal axis, kisspeptin was discovered in mammals. It is a hypothalamic neuropeptide that acts on kisspeptin receptors expressed on GnRH neurons to stimulate its release, with gonadoinhibin having a direct effect on kisspeptin neurons [10].
Kisspeptins are a group of hypothalamic arginine-phenylalanine amide peptides encoded by the KISS1
on chromosome 1q32. Kisspeptin isoforms are formed by proteolytic enzyme cleavage of the 145 amino acid gene product to produce peptides of varying lengths, ranging from 54 to 10 amino acids, designated by a suffix (e.g., kisspeptin-54 consists of 54 amino acids) [11].
Studies [12, 13] have demonstrated that kisspeptin enhances the secretion of LH and FSH in women. The stimulating effect of this neuropeptide on HGNA has been identified in various physiological conditions, in different phases of the menstrual cycle, as well as during pregnancy and lactation. People with kisspeptin receptor mutations exhibit abnormal pulsatile secretion of LH, and deletion of kisspeptin receptor genes in mice and rats results in a complete absence of pulsatile secretion of this gonadotropin.
In the experiment, after pinealectomy and orbital enucleation, the expression of gonadoinhibin mRNA in the bird brain decreased, while the administration of melatonin increased the expression of mRNA. Melatonin receptors have been found to be expressed on GnRH neurons, indicating that melatonin induces the expression and release of this neuropeptide by acting directly on its neurons, which has been confirmed in experimental models [9].
According to the results of the study by S.Yu. Vorotnikova et al. [14], the kisspeptin level in healthy women of reproductive age reaches 13.7 ng/ml. Data on the leading role of kisspeptin in the regulation of GGNA substantiate the advisability of using its analogues for disorders in the reproductive system associated with its deficiency, as well as in ART programs. Kisspeptin analogues may have therapeutic potential similar to GnRH agonists, acting as an ovulation inducer for the prevention of ovarian hyperstimulation syndrome [15]. However, in patients with POI, kisspeptin as a clinical marker is not strictly specific.
One of the first manifestations of SOR and hypergonadotropic hypogonadism are neurovegetative disorders characterized by hot flashes, sweating, and “night sweats,” which can significantly reduce the quality of life of patients. The second serious complaint of patients with ODS is infertility associated with chronic anovulation and low oocyte quality [16].
The drug pineamin, which includes a complex of low-molecular-weight water-soluble polypeptide fractions isolated from the pineal gland of cattle, is currently available on the Russian market. It has a stimulating effect on the synthesis of endogenous melatonin by stimulating the release of gonadoinhibin. The drug is indicated for the relief of vasomotor symptoms, however, in preclinical studies in experimental models of age-related decline in fertility, pineamin was shown to improve reproductive function, possibly by improving follicular quality and reducing follicular atresia.
primary goal
research - assessment of the clinical effectiveness of the drug pineamin, GEROPHARM LLC (Russia)" in patients with SOR and POI.
Pineamin lyophilis for the preparation of intramuscular solution 10 mg bottle N 10
Dosage form
Lyophilisate for preparation. solution for intramuscular administration of 10 mg: 10 pcs.
Release form, packaging and composition of the drug
Lyophilisate for the preparation of a solution for intramuscular administration in the form of a powder or porous mass of white or white with a yellowish tint.
bovine pineal gland polypeptides 10 mg
a complex of water-soluble polypeptide fractions of the epiphysis (pineal gland) of cattle not older than 12 months of age, isolated from dry Pineamin® extract
Pharmacotherapeutic group:
Antimenopausal agent
pharmachologic effect
The active ingredient of the drug Pineamin® is a complex of low molecular weight water-soluble polypeptide fractions isolated from the pineal gland of cattle.
The drug optimizes the epiphyseal-hypothalamic relationship, normalizes the function of the anterior pituitary gland and the balance of gonadotropic hormones.
In experiments on laboratory animals with age-related decline in fertility in groups receiving the drug Pineamin®, there was a tendency to increase libido and increase reproductive capacity, and a slight calming effect on the psycho-emotional state of animals was noted.
In a double-blind, placebo-controlled study of the drug in a population of postmenopausal women, a significant decrease in the severity of menopausal disorders was found according to the Kupperman index due to the positive effect of the drug on the neurovegetative manifestations of menopausal syndrome, such as hot flashes, hyperhidrosis, headaches, and palpitations at rest. , increased excitability, sleep disturbances.
Toxicological studies have proven the non-toxicity of Pineamin®. When studying acute toxicity, it was not possible to achieve lethal effects when using the drug in maximum doses exceeding the therapeutic dose for humans by 10,000 times. Subacute (30 days) and chronic (90 days) daily intramuscular administration of the drug Pineamin® to experimental animals did not have a negative effect on the main systems (nervous, cardiovascular, hematopoietic, urinary and respiratory), as well as on metabolism and general condition body.
Pharmacokinetics
The composition of the drug Pineamin®, the active substance of which is a complex of polypeptide fractions, does not allow for conventional pharmacokinetic analysis of individual components.
Indications for the drug Pineamin®
— neurovegetative disorders in menopausal syndrome in women with contraindications to hormone replacement therapy (3HT) or refusal to carry it out.
Dosage regimen
The drug is administered intramuscularly.
Before injection, the contents of the bottle are dissolved in 1-2 ml of a 0.5% solution of procaine (novocaine), water or 0.9% sodium chloride solution and administered once daily at a dose of 10 mg for 10 days.
If necessary, repeat the course after 3-6 months.
If an injection is missed, it is not recommended to administer a double dose, but to administer the next injection as usual on the scheduled day.
From the genital organs and breast: often (≥1%, but <10% of prescriptions) - bloody vaginal discharge.
From the skin and subcutaneous tissues: rarely (≥0.01%, but <0.1% of prescriptions) - infiltrate at the site of intramuscular administration of the drug.
If these side effects occur, the patient should consult a doctor.
If any of the side effects indicated in the instructions worsen or any other side effects not listed in the instructions occur, the patient should inform the doctor.
Contraindications for use
metrorrhagia (bloody discharge from the genital tract of unknown origin);
precancerous or malignant diseases, incl. estrogen-dependent tumors of the reproductive system and breast;
pregnancy;
breastfeeding period;
children and adolescents up to 18 years of age;
hypersensitivity to the components of the drug.
Use during pregnancy and breastfeeding
The drug is not intended for use during pregnancy and breastfeeding.
Use in children
The use of the drug is contraindicated in children and adolescents under 18 years of age.
special instructions
Pineamin® should be used only as directed by a physician.
The drug does not affect the concentration of sex hormones in the blood plasma. However, it is recommended to use it with caution in case of leiomyoma and endometriosis.
Due to the possibility of individual hypersensitivity to the components of the drug, it is recommended that a test injection be performed before starting therapy.
The drug solution must be prepared immediately before use. The prepared solution cannot be stored.
No special precautions are required when disposing of unused medicinal products.
Impact on the ability to drive vehicles and operate machinery
The effect of the drug on the performance of potentially hazardous activities that require increased concentration of attention and speed of psychomotor reactions has not yet been identified.
Overdose
No cases of overdose have been identified.
Symptoms: in case of overdose, bloody discharge from the vagina and an increase in the concentration of estradiol in the blood are possible.
Treatment: if symptoms of overdose occur, discontinuation of the drug and symptomatic therapy are necessary.
Drug interactions
Drug interactions with other drugs have not yet been identified.
Pharmaceutical incompatibility
The Pineamin® solution is not recommended to be mixed with other solutions.
Storage conditions for the drug Pineamin®
The drug should be stored in a place protected from light, out of reach of children, at a temperature of 2° to 20°C.
Shelf life of the drug Pineamin®
Shelf life: 3 years. Do not use after the expiration date stated on the package.
Terms of sale
The drug is available with a prescription.
Material and methods
A total of 66 patients aged 18 to 45 years who attended an outpatient appointment during 2018-2019, with a confirmed diagnosis of premature ovarian failure, ovarian syndrome and vasomotor symptoms, took part in the study.
Inclusion criteria: women aged 18-45 years with neurovegetative disorders, a decrease in the number of antral follicles, an FSH level >25 U/l, measured 2 times with an interval of 1 month in the 1st phase of the menstrual cycle. The presence of a preserved menstrual cycle or delayed menstruation of varying duration.
Exclusion criteria: pregnancy, lactation, individual intolerance to pineamin, hyperprolactinemia; hypothyroidism; other endocrine or systemic diseases that potentially affect the physiology of human reproduction, taking medications that can interfere with the normal function of the GGTN, as well as metformin, pineamin within 6 months preceding the study, severe liver and kidney diseases (serum creatinine 200 µmol/l and higher, serum potassium more than 5.0 mmol/l), stage III hypertension (BP 180/110 mm Hg), symptomatic arterial hypertension, coronary heart disease (CHD), acute cerebrovascular accident or transient cerebrovascular accident history of blood circulation, type 1 diabetes mellitus, insulin therapy, concomitant diseases that require additional therapy and make it difficult to assess the effectiveness and tolerability of the drug, smoking, bleeding from the genital tract of unknown etiology.
Study design.
All patients were divided into three groups: Group 1 included 22 women with contraindications to the use of estrogen-gestagen drugs, who received intramuscular injection of pineamin diluted in 1-2 ml of water for injection once daily at a dose of 10 mg for 10 days from the 5th to 15th day of the menstrual cycle or against the background of the absence of menstruation, regardless of the day of the cycle, for the correction of autonomic disorders; Group 2 included 22 patients who received hormonal replacement therapy with sex steroids using a drug containing 2 mg of estradiol and 10 mg of dydrogesterone, from the 1st day of the menstrual cycle or against the background of a long delay in the menstrual cycle with an endometrial thickness of less than 4 mm and pineamin according to the above scheme; The 3rd group, involving 22 patients, received only a drug containing 2 mg of estradiol and 10 mg of dydrogesterone, according to a similar regimen. Allocation to groups 2 and 3 was carried out using simple randomization.
All patients were discontinued from hormonal therapy (estrogen-gestagen drugs, melatonin) for 2 weeks. Patients who had not previously received therapy did not undergo a washout period.
The objectives of the study included assessing the effect of pineamin on the levels of LH, FSH, AMH, estradiol, kisspeptin and on the growth of antral follicles according to pelvic ultrasound data.
Participants were assessed for ovarian reserve parameters in the 1st phase of the cycle (before treatment and 1 month after inclusion in the study):
1. Study of the level of LH, FSH, estradiol, AMH, kisspeptin before the start of treatment and in the next cycle after the start of treatment on the 2-3rd day of the menstrual cycle.
2. Ultrasound of the pelvic organs on the 5-7th day of the menstrual cycle (or if menstruation is delayed) with counting the number of antral follicles and on the 21-23rd day of the menstrual cycle in the 1st treatment cycle.
Determination of the levels of LH, FSH, estradiol, AMH in the blood was carried out by chemiluminescence immunoassay using the automated Vitros 3600 system (Johnson & Johnson, USA). The content of kisspeptin-54 in serum samples was determined using commercial kits (USA) by enzyme-linked immunosorbent assay; optical density was measured using a 1420 Multilabel Counter VICTOR2 (Perkin Elmer).
Tolerability assessment criteria: excellent - no side effects or abnormalities in laboratory tests; good - the appearance of short-term mild side effects or insignificant deviations in laboratory tests, which do not require treatment adjustment; satisfactory - development of moderately severe side effects or significant deviations in laboratory tests, requiring treatment adjustment; bad - the development of moderate or severe side effects or significant deviations in laboratory tests requiring discontinuation of the drug.
Statistical analysis was carried out using Microsoft Excel 2016 (Microsoft corp., USA) and IBM SPSS Statistics (IBM corp., USA). The distribution of quantitative variables, as tested for normality using the Shapiro-Wilk test, differed from normal, so the median and interquartile range were calculated as descriptive statistics. Comparisons of quantitative variables in the three groups were performed using the Kruskal-Wallis test, followed by post hoc analysis using Dunn's test, adjusted for multiple comparisons. Analysis of changes in associated variables within groups (before and after treatment) was performed using the Wilcoxon test. Correlation analysis was performed using Spearman's rank correlation test. The probability of committing a type I error of less than 5% was considered statistically significant ( p
<0,05).
Pineamin®
PINEAMIN ®
Group name: polypeptides of the pineal gland of cattle. Dosage form: lyophilisate for the preparation of a solution for intramuscular administration. Composition: The drug PINEAMIN®, lyophilisate for the preparation of a solution for intramuscular administration of 10 mg, is a complex of water-soluble polypeptide fractions of the pineal gland (pineal gland) of cattle not older than 12 months of age, isolated from the dry extract of Pineamin (GEROPHARM LLC, Russia ), containing glycine as a stabilizer (Ajinomoto Co. Inc., Japan; Panreac, Spain) in a ratio of 1: 2. Description of the drug: Powder or porous mass of white or white with a yellowish tint. Pharmacotherapeutic group of the drug: Anticlimacteric. ATX code: G02CX. Pharmacodynamics: When taken, the drug optimizes the epiphyseal-hypothalamic relationship, normalizes the function of the anterior pituitary gland and the balance of gonadotropic hormones. In a double-blind, placebo-controlled study of the drug in a population of postmenopausal women, it was found that with its use, a significant decrease in the severity of menopausal disorders according to the Kupperman index occurs due to the positive effect of the drug on the neurovegetative manifestations of menopause. Previously, in preclinical studies on experimental animals, long-term (30 and 90 days) intramuscular administration of the drug Pineamin did not reveal its negative effects on the main systems (nervous, cardiovascular, hematopoietic, urinary and respiratory), as well as on metabolism and general condition body. Indications for use: Neurovegetative disorders in menopausal syndrome in women with contraindications to hormone replacement therapy (HRT) or refusal to carry it out. Contraindications: Hypersensitivity or intolerance to any of the components of the drug, children under 18 years of age, pregnancy and breastfeeding, metrorrhagia (bloody discharge from the genital tract of unknown origin). Precancerous and malignant diseases, including estrogen-dependent tumors of the reproductive system and breast. With caution: The drug does not affect the concentration of sex hormones in the blood plasma. However, it is recommended to use it with caution in cases of uterine leiomyoma and endometriosis. Use during pregnancy and breastfeeding: The drug is not indicated for use during pregnancy and breastfeeding. Method of administration and dosage: The drug is administered intramuscularly. Before injection, the contents of the bottle are dissolved in 1-2 ml of 0.5% solution of procaine (novocaine), water for injection or 0.9% sodium chloride solution and administered once daily at a dose of 10 mg for 10 days. If necessary, repeat the course after 3-6 months. If an injection is missed, it is not recommended to administer a double dose, but to administer the next injection as usual on the scheduled day. Side effects: Possible side effects of Pineamin are allergic reactions in case of individual hypersensitivity to the components contained in the drug. In the clinical study conducted, isolated cases of the development of such adverse reactions as (MedDra classification) were registered:
| MedDra class | Adverse reaction | Frequency (according to WHO classification) |
| Disorders of the genital organs and mammary glands | Bloody vaginal discharge | Common (≥ 1% but < 10% of prescriptions) |
| Skin and subcutaneous tissue disorders | Infiltration at the site of intramuscular injection of the drug | Rare (≥ 0.01% but < 0.1% of prescriptions) |
If these side effects occur, you should consult a doctor. If the phenomena indicated in the instructions worsen or other side effects not mentioned occur, you must inform your doctor. Overdose: No cases of overdose have been identified. Possible symptoms of a drug overdose are bloody vaginal discharge and an increase in the concentration of estradiol in the blood. In these cases, discontinuation of the drug and symptomatic therapy are necessary. Interaction with other drugs: Drug interactions with other drugs have not yet been identified. Incompatibility: PINEAMIN® solution is not recommended to be mixed with other solutions. Special instructions: PINEAMIN® should be used only as prescribed by a doctor! In view of the possibility of individual hypersensitivity to individual components contained in the drug, it is recommended to administer it as a test injection before starting therapy. The bottle with the drug solution cannot be stored or used after storage. No special precautions are required when disposing of unused medicine. Effect on the ability to drive vehicles and machinery: The effect of the drug Pineamin for medical use on the performance of potentially hazardous activities that require increased concentration and speed of psychomotor reactions has not yet been identified. Release form: Lyophilisate Pineamin for the preparation of solution for intramuscular administration 10 mg. Bottles made of colorless glass with a capacity of 5 ml, with aluminum caps with a tear-off plastic lining of pink or orange color with a raised inscription: “GEROPHARM”, 5 bottles each in a blister pack made of polyvinyl chloride film and aluminum foil. 1 or 2 blister packs with instructions for use in a cardboard pack. Storage conditions: In a place protected from light at a temperature of 2 to 20 ° C. Keep out of the reach of children. Shelf life: 3 years. Conditions for dispensing from pharmacies: Dispensed by prescription. Registration number of Pineamin: LP-003202 Manufacturer: GEROPHARM LLC, Russia 191144, St. Petersburg, Degtyarny lane, 11, lit. B Telephone (multi-channel) Fax Hotline telephone: 8-800-333-4376 (calls within Russia are free)
results
The characteristics and hormonal profile of the patients included in the study are presented in Table. 1.
Table 1. Characteristics and hormonal profile of study participants, median [lower quartile; upper quartile]
All participants did not differ statistically significantly in age and number of antral follicles. There were no statistically significant differences in the levels of LH, FSH and AMH among patients of the examined groups before and after treatment. But when comparing the indicators before and after treatment, significant changes were found in each individual group: a decrease in the level of LH and FSH, as well as an increase in estradiol (see Table 1, Table 2).
Table 2. Dynamics of estradiol and kisspeptin levels in patients of the examined groups, median [lower quartile; upper quartile] No statistically significant changes in AMH levels were recorded.
After treatment, in group 1 (pineamine monotherapy group) and group 2 (hormone replacement therapy in combination with pineamin), patients noted complete relief of vasomotor symptoms, while in group 3 (estrogen-progestogen drug) only 17 women noted a complete cessation of hot flashes 1 month after treatment. Tolerability of pineamin was excellent in 41 patients (no side effects were recorded), one participant complained of pain in the legs (group 2), and therefore ultrasound diagnostics of the vessels of the lower extremities was recommended. No deviations were detected according to Doppler sonography; subsequently, a course of treatment with diosmin was prescribed, which led to a positive dynamics of complaints.
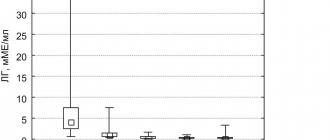
Before inclusion in the study, statistically significant differences in the level of kisspeptin in the groups were found; after treatment, no significant changes in this parameter were found between the groups, however, when analyzing changes in the kisspeptin indicator in each group before and after treatment, it was found that its level increased significantly in the combined group therapy (see Table 2).
Patients did not differ in terms of maximum follicle diameter (Dmax) before inclusion in the study (Table 3).
Table 3. Dmax before and after treatment in patients of the examined groups, median [lower quartile; upper quartile] After treatment, the diameter of the follicle in patients of the examined groups increased statistically significantly: the maximum diameter was achieved in the combination therapy group (2nd group), and the minimum - in the estrogen-gestagen drug therapy group (3rd group), the same a pattern was recorded when analyzing the ratio Dmax after treatment/Dmax basal (fold increase in follicle diameter) (see Table 3) - follicle growth of more than 4 times was achieved in the 2nd group. In some patients in the 2nd phase of the cycle, according to ultrasound data, the corpus luteum was visualized: in the 2nd group - in 13 patients, in the 3rd group - in 1 patient, in the 1st group the corpus luteum was not detected in any of the patients. participants
In a correlation analysis, no correlations were found between kisspetin levels and other hormonal indicators of ovarian reserve. Correlations between LH and FSH levels were recorded ( r
=0.77;
p
<0.001), LH and AMH (
r
=–0.306;
p
=0.012).
Menopausal syndrome: innovations in menopausal therapy
The climacteric period (CP) is a physiological period in a woman’s life, during which, against the background of age-related changes, a gradual decrease and shutdown of ovarian function and cessation of estrogen production occur [1–3]. During this period of complex age-related restructuring of the body associated with the decline of the reproductive system, the quality of life changes significantly [4]. KP is typical for both women and men, but in the latter it occurs later and develops more slowly. KP is often referred to as the menopausal “transition.” In women, it occurs between the ages of 45 and 55 years; the time of occurrence of CP depends mainly on the genetic characteristics of the body [5]. It has been established that in many women who smoke, menopause occurs 1–2 years earlier, since smoking products destroy the follicular apparatus of the ovaries [6]. There are 3 phases of CP: premenopause, menopause, postmenopause [1, 7]. Premenopause begins after 45 years of age and lasts from 2 to 6 years. Postmenopause is the time period from menopause to the permanent cessation of ovarian hormonal function. Perimenopause is the period from the onset of menstrual irregularities and vegetative-vascular symptoms to 2 years after the cessation of the last menstruation, called menopause. Menopause is determined retrospectively - after 12 months. after the last menstruation. During perimenopause, a decrease in immune defense gradually progresses, non-infectious morbidity increases, meteolability increases, osteoporosis develops, degenerative changes in the cardiovascular system progress, metabolic disorders occur, and body weight increases due to adipocyte hyperplasia [8]. It has been established that the process of follicle apoptosis accelerates significantly after 37 years. The reason for this is considered to be a decrease in the synthesis of inhibin in the granulosa cells of the follicles, which is the main regulator of the synthesis of follicle-stimulating hormone (FSH) in the pituitary gland. An increase in FSH levels is noted long before perimenopause with unchanged levels of luteinizing hormone (LH) and E2 hormone in the blood [9–11]. KP is often complicated by climacteric syndrome (CS). CS is a complex of vegetative-vascular, mental and metabolic-endocrine disorders that occur in women against the background of fading hormonal function of the ovaries and general age-related involution of the body. The most common early manifestations of CS are vegetative-vascular, which occur in 40–80% of women in the CS [12]. In case of CS, there are 3 types of disorders: – vegetative-vascular: hyperhidrosis, headaches, hot flashes, chills, dizziness, tachycardia, sympathoadrenal and vagoinsular crises; – emotional and mental: irritability, drowsiness, anxiety, depression, decreased mood, attention, memory impairment; – metabolic-endocrine: medium-term (urogenital symptoms, changes in the skin and its appendages) and late (cardiovascular diseases, osteoporosis). The average duration of vegetative-vascular disorders is 7.4 years, which significantly affects the health of women, increasing the risk of cardiovascular diseases, as well as metabolic and endocrine disorders [13]. The most common symptom of CS is hot flashes - their frequency is about 75%. Night sweats are also a common symptom with an incidence of up to 67%. Other main and frequently occurring symptoms include: insomnia, weight gain, mood swings, increased fatigue, atrophic vaginitis, joint pain, headaches [12, 14]. The main cause of CS is estrogen deficiency, which subsequently leads to dysfunction of the hypothalamic-pituitary system.
Menopausal hormone therapy
Menopausal hormone therapy (MHT) plays a significant role in the treatment of CS. MHT improves a woman’s quality of life, helping to prevent a number of complications from various organs and systems, pharmacologically replacing the hormonal function of the ovaries in women with deficiency of sex hormones. Indications for the use of MHT are: the presence of vasomotor and psychovegetative climacteric disorders associated with estrogen deficiency, genitourinary disorders associated with atrophic processes in the lower genitourinary tract, prevention of osteoporosis and fractures, premature, early and artificial menopause [15]. There are absolute and relative contraindications to the use of MHT. The absolute ones include: bleeding from the genital tract of unknown origin, breast and endometrial cancer, acute hepatitis, acute deep vein thrombosis, acute thromboembolism, allergy to the components of the drug, cutaneous porphyria. Relative contraindications include: uterine fibroids, endometriosis, venous thrombosis and embolism, familial hypertriglyceridemia, cholelithiasis, epilepsy, and a history of ovarian cancer [15]. Modern methods of treating CS are usually divided into hormonal (systemic and local therapy) and non-hormonal methods. Hormonal agents include oral estrogens, gestagens, transdermal estrogens, and combination drugs (estrogens + progestogens). Preference is given to low- and micro-dose hormonal contraceptives, especially in menopausal women. Before prescribing MHT, it is necessary to conduct an examination: a study of the level of blood hormones (FSH, LH, prolactin, thyroid-stimulating hormone (TSH)), an examination of the condition of the cervix and mammary glands (cytological screening, mammography), ultrasound of the pelvic organs and other methods in accordance with the indications . There are also alternative (non-hormonal) methods of treating CS: the prescription of herbal remedies, dehydroepiandrosterone, nutritional supplements, the drug Klimalanin, aminophenylbutyric acid, tranquilizers, homeopathic drugs, both individually and in various combinations. The main types of MHT medications include those containing estrogens. The advantages of oral estrogens are that they are easy to use and have a positive effect on various menopausal disorders and dyslipoproteinemia. It should be borne in mind that complications and adverse effects are possible when using MHT. For example, in case of gastrointestinal diseases, incomplete absorption of the drug is possible; large doses are required to achieve an effect. Oral estrogens undergo active metabolism in the liver, and increased concentrations of estrogens in the liver can stimulate the synthesis of various biological active substances (clotting factors, angiotensin, sex hormone binding globulin (SHBG)). Patients may sometimes not respond to oral MHT. Taking oral estrogens predisposes to the formation of gallstones. Side effects of different types of MHT are presented in Table 1.
In view of this, against the background of MHT, dynamic monitoring, monitoring of blood pressure, drug tolerance, and identification of possible complications and side effects are necessary. Treatment methods for CS are mainly aimed at replenishing estrogen deficiency, normalizing the function of the hypothalamic-pituitary-ovarian system and are most often used for a long time - usually from 2 to 10 years. Many scientists have long thought about the possibility of creating a drug that would affect various parts of the reproductive system and would be safer than MHT, so that its use would be much shorter, but no less effective. Based on the significant role of endogenous melatonin in regulating the function of the reproductive system, scientists put forward the idea of creating a drug based on pineal gland polypeptides that restore the functional density of pinealocytes, the secreting cells of the pineal gland [16]. As is known, the pineal gland, located in conjunction with the hypothalamus in the brain, is the main source of endogenous melatonin. The role of endogenous melatonin in the regulation of the reproductive system is the activation of gonado-inhibitory neurons, also located in the hypothalamus. Gonadoinhibin (GnIH) was first described by Tsutsui K. et al. in 2000 [17]. Gonadoinhibin inhibits the secretion of GnRH in the hypothalamus and has an inhibitory effect on the synthesis of kisspeptin in the neurons of the same name in the hypothalamus [18, 19]. Scientists from St. Petersburg synthesized a complex of low molecular weight water-soluble polypeptide fractions PPG (Polypeptides of Pineal Gland), isolated from the pineal gland of cattle [20, 21]. The effect of the resulting complex on the reproductive system of women was established, expressed in a decrease in psycho-emotional stress and vegetative-vascular disorders, which is especially significant for women with CS [22]. The new original drug Pineamin® was created by synthesizing a complex of pineal gland polypeptides. The results of numerous studies have shown that the advantages of this drug are the rapid relief of neurovegetative symptoms of CS and the absence of toxicity [23]. When using it, no negative effects on the nervous, hematopoietic, cardiovascular system and metabolism were detected. One of the features of Pineamin is the absence of a proliferative effect on target organs (cervix, endometrium) even with long-term use [24]. Subsequently, a large clinical study was conducted in large centers in Russia to study the effectiveness of the drug, the result of which was the identification of its high effectiveness and safety when used in patients with CS, mainly with vegetative-vascular and psychoneurological disorders [23]. The proliferative effect of the drug was assessed. After a detailed examination, 120 patients were selected and divided into 3 groups of equal numbers: 1st – patients taking placebo, 2nd – Pineamin® (1 course), 3rd – Pineamin® (2 courses). All patients were assessed for endometrial thickness by ultrasound before treatment, after 90 and 180 days of use. Endometrial thickness did not statistically significantly increase in 3 groups - in the placebo group and the groups receiving Pineamin® (p<0.05). Clinically significant deviations in the condition of the endocervix according to Papanicolaou smears from the cervical epithelium were not detected in any of the patients. During the study, indicators of the blood coagulation system (fibrinogen, PTI, ATPV and INR) were assessed. According to the results of the study, the drug Pineamin® had no effect on her parameters, and their levels were within normal limits and corresponded to the level at the time of inclusion of patients in the study. Thus, Pineamin® can be considered safe with respect to its effect on the blood coagulation system. The indicators of the blood lipid profile over time were studied. The initial indicators of lipid metabolism did not differ significantly from those after 180 days of taking Pineamin (p<0.04). This led to the conclusion that Pineamin does not affect the lipid profile; there is no need for additional laboratory monitoring. When assessing the safety of the drug Pineamin® during the study, 22 cases of adverse events (AEs) were identified in both groups (placebo and Pineamin®). There were no statistically significant differences in the incidence of AEs between groups. Almost all cases of AEs [22] were regarded as non-serious and completely reversible (ARVI, glossitis, menstrual-like reaction, transient increase in estradiol levels, infiltration at the injection site). One serious AE in the form of acute coronary syndrome was noted in the placebo group after 3 months. after finishing the injections. No statistical patterns associated with receiving a course of Pineamin injections have been identified. Pineamin® can be used in combination with MHT, as well as for the treatment of other somatic and functional disorders, since its negative interaction with any drugs has not been established.
Conclusion
The symptoms experienced by women in KP have a significant impact on quality of life. In modern medicine, the choice of treatment method for patients with CS is extremely relevant. The drug Pineamin® has a low number of side effects and minimal impact on organs. Its use as an independent therapy or in combination with other treatment methods opens up new opportunities for doctors in the treatment of such a complex pathological condition as CS. A preliminary analysis of the practical experience of using the drug in 450 women with CS in various cities of Russia revealed its effectiveness, a decrease in pronounced neurovegetative and psycho-emotional symptoms with good tolerability. Thus, a new class of medicine with unique capabilities for treating patients with CS has appeared in the arsenal of doctors. Research continues.
Discussion
The female reproductive system is under the control of GnRH: pulsatile releases of GnRH and the resulting secretion of gonadotropins are responsible for puberty and support the cyclic function of the ovaries in adulthood. Tonic (continuous at a relatively low, basal level) secretion of GnRH, LH and FSH is regulated by the principle of negative feedback by estrogens. In the last decade, many studies have shown that kisspeptin is the highest regulatory element of the pulsatile and spasmodic release of GnRH, which is involved in the onset of puberty, sexual differentiation of the brain, ovulation and reproductive function. In mammals, two populations of kisspeptin neurons have been found that play different functional roles, positive and negative, in response to estrogen exposure [17].
In humans and mice, inactivating mutations in kisspeptin lead to hypogonadotropic hypogonadism. New evidence indicates a potential role for hypothalamic neuropeptide in modulating the activity of various systems in the brain and many peripheral organs. Several studies have shown that kisspeptin and its receptor are expressed in various tissues and can have direct autocrine-paracrine effects, including in the reproductive organs: ovary, placenta [18].
Kisspeptin levels increase from the early follicular to preovulatory phase and from the preovulatory to luteal phase [19]. Neuropeptide concentrations were found to peak on day 11, when the dominant follicle was approximately 1.2 cm, explaining its role as a potential regulator of folliculogenesis and predictor of ovulation [20]. Circadian rhythms, including the melatonin system, influence the seasonality of reproduction in animals. Melatonin has been found to inhibit kisspeptin expression. Although in vivo
showed that kisspeptin neurons do not have melatonin receptors [21–23].
Recent work has established an association between low kisspeptin levels and unexplained infertility, poor egg quality and decreased likelihood of fertilization, thin endometrium and impaired blastocyst implantation [24]. A number of animal studies indicate the role of this neuropeptide system and its receptors in the ovary, and their defects lead to POF. In mice with a haploinsufficient allele (an allele characterized by incomplete dominance, i.e., in the heterozygous state, it is not fully manifested in the phenotype) of the kisspeptin receptor, an early decrease in the frequency of ovulation, progressive loss of oocytes and antral follicles, a decrease in the number of preantral follicles, and decreased fertility were recorded. Atrophic processes, lack of follicular growth and maturation of the corpus luteum were detected in the ovarian tissue of these mice. This phenotype is associated with low levels of kisspeptin receptor mRNA expression in the ovary and lack of response to gonadotropin administration [25, 26].
According to our study, patients who received combination therapy with an estrogen-progestin drug and pineamine demonstrated the best indicators of follicular growth dynamics: the diameter of the follicles increased 4 times, while in 59% of patients a full-fledged corpus luteum was formed. Taking into account the mechanism of action of the drugs, all women showed a statistically significant decrease in LH and FSH, but we did not find any dynamics in the level of AMH, which can probably be explained by the high level of gonadotropins and the short period of observation of patients (1 month).
The mechanism of action of pineamin is not only the normalization of the work of GGNA, but it also acts as a trigger for the synthesis of its own melatonin, which indirectly can normalize the work of the kisspeptin system and its receptors in the ovaries, and this hormone is directly involved in folliculogenesis, stimulating the maturation of eggs , resulting in more corpora lutea in the combination therapy group. While taking an estrogen-gestagen drug, the patients expectedly showed an increase in the level of estradiol, which, based on the principle of negative feedback, had a suppressive effect on the level of gonadotropins.
An additional mechanism of action of pineamin is the activation of gonadoinhibin, which in turn blocks GnRH and reduces the activity of the kisspeptin system. However, in our patients, after treatment with pineamin and an estrogen-progestin drug, an increase in the level of the hypothalamic neuropeptide kisspeptin was recorded, which seems difficult to assess the clinical significance of the results obtained, since the initial groups were not comparable in terms of the level of this indicator, and there is not a sufficient number of publications in which normative intervals for it would be precisely established, along with the various analytical methods used to measure it [27]. In this regard, it can be assumed that the determination of kisspeptin levels in patients with POF is low informative and clinically unreasonable.