According to a survey conducted by ROSSTAT “Comprehensive monitoring of living conditions of the population 2018”, infectious diseases are the third reason for the independent use of medications as part of self-medication and self-prevention [1]. In such a situation, the pharmaceutical worker is actually the only qualified interlocutor of the buyer who is faced with a choice. Therefore, today, competent pharmaceutical consulting is gaining more and more importance.
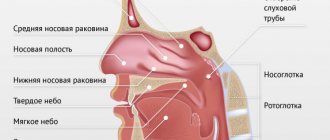
The nasal mucosa is a typical “entry gate” for respiratory viruses, therefore, the temporary guidelines of the Russian Ministry of Health for the prevention, diagnosis and treatment of the new coronavirus infection (COVID-2019) dated March 27, 2020 indicate that for nonspecific prevention of coronavirus infection it is recommended to use drugs for topical use with barrier functions. Such drugs and medical devices form a physical barrier on the mucous membrane of the nasal cavity, preventing the adhesion of the virus. The compounds that form the barrier are gradually eliminated due to the work of the mucociliary apparatus. Theoretically, the protective layer will help slow the progression of the disease, since there will be fewer cells available to attack by viruses.
Directions for use and doses
Intranasally, as necessary, periodically, when visiting crowded places (to avoid airborne infection).
In adults and children (under adult supervision): 1 spray in each nasal passage 3-4 times a day (every 6-8 hours) - as a rule, the recommended dosage is sufficient for protection throughout the day.
It is recommended to repeat spraying the drug after each blowing of the nose to resume the formation of a protective film.
MEDICATIONS
The barrier function in medicinal products is performed by the substances that make up their ointment or gel base. In addition, intranasal medications also contain active ingredients: dioxotetrahydroxytetrahydronaphthalene (“Oksolin”, nasal ointment 0.25%), interferon alpha-2b (“Viferon”, ointment and gel for external and local use; “Infagel” gel for external and local use). However, the effectiveness of their use as declared antiviral agents does not have a sufficient scientific basis.
| Trade name | Excipients | Active ingredients | With caution / Contraindications |
| Oksolin, nasal ointment 0.25% | Liquid paraffin (vaseline oil), petroleum jelly | Dioxotetrahydroxytetrahydronaphthalene | With caution during pregnancy, breastfeeding, contraindication - children under 2 years of age |
| Viferon, gel for external and local use | Alpha tocopherol acetate, methionine, benzoic acid, citric acid monohydrate, sodium tetraborate decahydrate, sodium chloride, human albumin, distilled glycerin (glycerol), sodium carmellose, ethanol 95%, purified water | Interferon alpha-2b human recombinant | Pregnancy and breastfeeding are not a contraindication for use due to the very low absorption of components; use in newborns is possible |
| Viferon, ointment for external and local use | Alpha tocopherol acetate, lanolin anhydrous, medical petrolatum, peach oil, calcium pantothenate, sodium benzoate, human albumin, sodium acetate trihydrate, sodium chloride, disodium edetate dihydrate, purified water | Interferon alpha-2b human recombinant | Possible use during pregnancy and breastfeeding due to very low absorption of components, contraindication - children under 1 year of age |
| Infagel, gel for external and local use | Aluminum hydroxide, polyvinyl alcohol, purified water | Interferon alpha-2b recombinant | Contraindicated for persons suffering from allergic diseases in the acute stage |
special instructions
It is recommended to use the drug in advance, 10–15 minutes before intended trips on public transport, plane, train, etc., as well as before visiting crowded places.
It is recommended to shake the bottle before each use!
How the product works
1. When using for the first time, it is recommended to adjust the dosing device to obtain the optimal dose. To do this, you need to place the dispenser bottle opposite you and, lightly pressing the walls of the bottle, make 2 test injections into the air - a stream of white powder should appear.
2. Before use, if necessary, you should hygienically clean the nasal cavity or blow your nose.
3. Pinch one nasal passage with your finger.
4. Place the spout of the bottle in the opposite nasal passage and make 1 spray while inhaling, pressing on the walls of the bottle.
5. Hold your breath for 2 seconds and then take a deep, calm breath so that the powder penetrates the nasal passage.
6. Repeat the procedure with the other nostril.
The drug can be used as often as necessary. Re-use is recommended after each nasal cleansing.
Do not use simultaneously with other drugs for the nose, because this may reduce the effectiveness of Nazaval Plus.
In case of contact with eyes, it is recommended to rinse them with water.
Do not use if the bottle is damaged.
Nazaval Plus nasal spray 500 mg 200 DOZES
Compound
Active ingredients: micronized cellulose of plant origin, wild garlic extract. Excipients: natural peppermint extract.
Indications for use
Nazaval® PLUS is used for the prevention of colds, ARVI (including influenza):
- once: before possible contact with a patient with ARVI, when visiting crowded places as a means of emergency protection;
- daily: during the ARVI season.
Nazaval® PLUS can be used in children and adults, as well as women during pregnancy and breastfeeding.
Contraindications
- individual intolerance to components;
- allergic reactions to garlic;
- tendency to nosebleeds.
Directions for use and doses
Children and adults: one spray into each nasal passage 3-4 times a day (every 5-6 hours).
Typically, this frequency of application is sufficient for protection throughout the day. If necessary, Nazaval® PLUS can be used as often as necessary. It is recommended to use Nazaval® PLUS 10–15 minutes before intended trips on public transport (plane, trains, etc.), when visiting crowded places. Application procedure:
Step 1. Before use, if necessary, hygienically clean the nasal cavity or blow your nose.
Step 2. Gently shake the bottle. When using for the first time, it is recommended to make 1 or 2 test injections into the air.
Step 3. Pinch one nasal passage with your finger, inhale the powder into the opposite nasal passage, pressing INTENSIVELY on the walls of the bottle.
Repeat the same procedure on the other side.
Storage conditions
Store in a dry place at a temperature not exceeding 25 °C. Keep out of the reach of children.
Best before date
3 years.
Do not use after the expiration date stated on the package. The bottle is recommended to be used within 6 months after first opening.
Do not use if the bottle is damaged.
special instructions
The safety of Nazaval® PLUS is due to the lack of interaction with organs and tissues of the body.
Nazaval® PLUS should be used in children under adult supervision. Before each use, it is recommended to hygienically clean the nasal cavity. Contact of the bottle spout with the nasal mucosa should be avoided. This may cause the bottle spout to become clogged with powder. If this does happen, clean the bottle spout with a thin, sharp object (needle, toothpick). If it is necessary to use it together with other nasal medications, Nazaval® PLUS should be used no earlier than 30 minutes after their use, having first cleared the nasal passages.
It is not recommended to use Nazaval® PLUS after using nasal ointments and oil-based nasal drops. If Nazaval® PLUS gets into your eyes, it is recommended to rinse them with water.
Description
Nazaval® PLUS acts as a natural barrier on the nasal mucosa and is used to protect against the penetration of viruses and bacteria that enter the upper respiratory tract when air is inhaled. It is a preventative against ARVI (including influenza) and other colds.
Dosage form
Fine powder of white or almost white color with a slight mint odor.
Use in children
The drug is not contraindicated in childhood and, after consultation with a doctor, can be used even in infants. In order for the medicine to have the expected effect, its administration into the nasal passages in children must be supervised by adults.
Action
The powder of cellulose and wild garlic extract from the dispenser enters the nasal mucosa and forms a transparent, gel-like, protective layer that does not interfere with breathing.
The gel-like layer is an effective barrier against viruses and bacteria, protecting the body from colds and ARVI. Due to its antiseptic properties, wild garlic extract inhibits the growth and neutralizes bacteria and viruses that enter the nasal mucosa with inhaled air.
Nasal spray, dosed Nazaval® PLUS is a barrier agent and does not have a systemic or local effect.
Use during pregnancy and breastfeeding
Nazaval® PLUS can be used during pregnancy and breastfeeding, since it does not have a systemic effect and does not contain preservatives.
Impact on the ability to drive vehicles and operate machinery
The use of the barrier agent Nazaval® PLUS does not affect the ability to drive vehicles and does not cause drowsiness.
MEDICAL DEVICES
"Nazaval PLUS"
“Nazaval PLUS” is a finely dispersed cellulose powder, which, when it enters the nasal cavity, binds to mucus and forms a gel-like layer on the mucous membrane that prevents viruses from entering the body. Due to wild garlic extract (allicin and ajoenes), the growth of bacteria and viruses is suppressed, and they are also neutralized [2].
The medical product “Nazaval PLUS” can be used in children and adults, as well as women during pregnancy and breastfeeding.
| Emergency protection | Seasonal protection |
| Before contact with a sick person, in crowded places, in public transport | For daily protection against respiratory viruses all day long |
| 1 injection into each nasal passage while inhaling, once (10-15 minutes before contact, visiting a public place, traveling) | 1 injection into each nasal passage while inhaling 3-4 times a day (every 5-6 hours) |
Natural peppermint extract included in Nazaval PLUS serves as an indicator of proper use. The appearance of a slight mint smell after injection indicates that the powder from the bottle has entered the nasal cavity.
Method of application:
Step 1. Before use, if necessary, hygienically clean the nasal cavity or blow your nose.
Step 2. Gently shake the bottle. When using for the first time, it is recommended to make 2 test injections into the air.
Step 3. Pinch one nasal passage with your finger, inhale the powder into the opposite nasal passage, pressing INTENSIVELY on the walls of the bottle. Repeat the same procedure on the other side.
It is recommended to reuse after each cleansing of the nasal cavity to restore the protective layer.
If it is necessary to use it together with other nasal medications, Nazaval PLUS should be used no earlier than 30 minutes after their use, having previously cleaned the nasal passages. It is not recommended to use Nazaval PLUS after using nasal ointments and oil-based nasal drops.
Also among buyers, the demand for medical products used to protect the nasal mucosa from aeroallergens has increased significantly, since the principle of action is the same.
"Nazaval"
The medical product “Nazaval” available on the pharmaceutical market, like “Nazaval PLUS”, contains fine cellulose powder, but without wild garlic extract. Recommended for protecting the nasal mucosa from allergens and pollutants, as well as other aggressive environmental factors inhaled with air. "Nazaval" can be used in children and adults, as well as women during pregnancy and breastfeeding.
Tafen nasal spray naz 50 mcg/dose cor 200 doses x1
Tafen nasal spray naz 50 mcg/dose 200 doses x1, ATX code: R01AD05 (Budesonide) Active substance: budesonide Rec.INN registered by WHO
Dosage form
TAFEN® NASAL
dosed nasal spray 50 mcg/1 dose: vial. 200 doses per dosage. devicereg. No.: P N014740/01 dated 12/19/08 - Indefinitely
Release form, composition and packaging
Nasal spray dosed in the form of a homogeneous suspension of white or almost white color.
1 dose
budesonide 50 mcg
Excipients: methyl parahydroxybenzoate - 50 mcg, propyl parahydroxybenzoate - 10 mcg, microcrystalline cellulose and carmellose sodium - 550 mcg, polysorbate 80 - 50 mcg, simethicone emulsion - 50 mcg, propylene glycol - 5 mg, sucrose - 15 mg, disodium edetate - 5 mcg, hydrochloric acid - 10 mg, water - 35.725 mg.
Clinical-pharmacological group: GCS for local use Pharmaco-therapeutic group: Glucocorticosteroid for local use
pharmachologic effect
GCS for intranasal use. It has a pronounced anti-inflammatory and antiallergic effect. When used in therapeutic doses, it has virtually no resorptive effect. It does not have mineralocorticoid activity and is well tolerated during long-term treatment. The drug has an inhibitory effect on the release of mediators of the inflammatory response, increases the synthesis of anti-inflammatory proteins, reduces the number of mast cells and eosinophilic granulocytes. Budesonide reduces the release of toxic proteins from eosinophils, free radicals from macrophages and lymphokines from lymphocytes. It also reduces the binding of adhesion molecules to endothelial cells, thus reducing the flow of leukocytes to the site of allergic inflammation. Budesonide increases the number of β-adrenergic receptors in smooth muscle. The drug inhibits the activity of phospholipase 2A, which leads to inhibition of the synthesis of prostaglandins, leukotrienes and PAF, which induce an inflammatory response. Budesonide also inhibits histamine synthesis, which leads to a decrease in histamine levels in mast cells.
Tafen® nasal reduces the severity of symptoms in allergic rhinitis, suppresses the late and early phases of the allergic reaction and reduces inflammation in the upper respiratory tract. Improvement in condition is observed 2-3 days after the start of treatment.
Pharmacokinetics
Suction
After inhalation of 400 mcg of budesonide, Cmax in plasma is achieved within 0.7 hours and is 1 nmol/l.
Only about 20% of the intranasally administered dose enters the systemic circulation.
Distribution
Due to good distribution in tissues and binding to plasma proteins, Vd is 301 l.
Metabolism
Systemic bioavailability of budesonide is low because more than 90% of the absorbed drug is inactivated in the process of one-step metabolism in the liver. Glucocorticoid activity of metabolites does not exceed 1%.
Removal
Metabolites are excreted mainly in urine (70%) and feces. T1/2 is 2-3 hours.
Indications
– prevention and treatment of seasonal and year-round allergic rhinitis,
- non-allergic rhinitis,
- nasal polyps.
ICD-10 codes
Dosage regimen
At the beginning of therapy, adults and children over 6 years of age are prescribed 2 doses (50 mcg of budesonide) in each nostril 2 times a day. The usual maintenance dose is 1 dose in each nostril 2 times a day or 2 doses in each nostril 1 time a day, in the morning. The maintenance dose should be the lowest effective dose to relieve rhinitis symptoms.
The maximum single dose is 200 mcg (100 mcg in each nostril), the maximum daily dose is 400 mcg for no more than 3 months.
For full therapeutic effect, regular and correct use is required.
If a dose is missed, it should be taken as soon as possible, but not less than 1 hour before taking the next regular dose.
Side effect
From the respiratory system: irritation of the mucous membrane of the nose and throat, nosebleeds, cough, less often dry nasal mucosa, sneezing.
Dermatological reactions: dermatitis, urticaria, rash are noted.
Other: fatigue, dizziness.
In exceptional cases, when using nasal corticosteroids, perforation of the nasal septum, angioedema, loss of smell, tachycardia, and growth retardation were observed.
When using the drug, side effects develop very rarely and are transient.
Contraindications for use
- fungal, bacterial and viral infections of the respiratory tract,
- active form of pulmonary tuberculosis,
- hypersensitivity to budesonide or any other component of the drug.
Use during pregnancy and breastfeeding
The use of Tafen® nasal during pregnancy is allowed only if the expected benefit to the mother outweighs the possible risk to the fetus.
If it is necessary to prescribe the drug during lactation, breastfeeding should be stopped.
Use in children
The drug is prescribed to children over 6 years of age.
special instructions
When switching from treatment with systemic corticosteroids to treatment with a nasal spray, due to the risk of developing adrenal insufficiency, caution is required during the period of restoration of the function of the hypothalamic-pituitary-adrenal system.
Since corticosteroids slow down wound healing, caution should be exercised when prescribing Tafen® nasal to patients who have recently undergone trauma or nasal surgery.
For a full therapeutic effect for allergic rhinitis, you need to take the drug regularly.
It is recommended to avoid contact with eyes.
Impact on the ability to drive vehicles and operate machinery
Tafen® nasal does not affect the ability to drive a car or operate machinery.
Overdose
Accidental overdose of Tafen® nasal does not cause any obvious symptoms. Acute overdose is unlikely.
With prolonged use of high doses, as well as with simultaneous use of other corticosteroids, symptoms of hypercortisolism may appear.
In this case, the drug should be stopped, gradually reducing its dose.
Drug interactions
The simultaneous use of Tafen® Nasal with inducers of microsomal oxidation (phenobarbital, phenytoin, rifampicin) may reduce the effectiveness of the former.
Methandrostenolone, estrogens, and ketoconazole enhance the effect of budesonide.
Storage conditions and periods
The drug should be stored out of the reach of children at a temperature not exceeding 25°C. Shelf life – 2 years.
Conditions for dispensing from pharmacies
The drug is available with a prescription.