Compound
Nasal spray:
- active ingredient xylometazoline hydrochloride - 0.50 mg or 1.00 mg;
- excipients: disodium edetate, sodium chloride, benzalkonium chloride, potassium dihydrogen phosphate, sodium hydrogen phosphate dodecahydrate, purified water up to 1 ml.
Nasal drops:
- active ingredient xylometazoline hydrochloride - 0.50 mg or 1.00 mg;
- excipients: benzalkonium chloride, thyrinodium edetate, monopotassium phosphate, dibasic sodium phosphate, sodium chloride, purified water up to 1 ml.
Composition and dosage forms
Rinostop is a medicine based on xylometazoline hydrochloride, a liquid solution for nasal use, transparent, without a distinct odor. Available in the form of drops and spray:
- for adults: liquid containing 0.1% active substance;
- for children 2–6 years old: solution with a concentration of 0.05%.
Excipients: water, sodium chloride, potassium dihydrogen phosphate, thyrinodium edetate.
The drug is packaged in plastic bottles of 10, 15, 20, 30 ml, equipped with drip or aerosol dispensers.
Pharmacodynamics and pharmacokinetics
The active substance of Rinostop, xylometazoline, belongs to the group of alpha-adrenergic agonists with local vasoconstrictor effects. Alpha adrenergic agonists are drugs that stimulate alpha adrenergic receptors , located in the walls of blood vessels and reacting to adrenaline , norepinephrine and other substances with a similar effect.
When applied to the mucous membrane of the nasal cavity, the drug causes a narrowing of the blood vessels located in it, but is almost not absorbed into the blood and does not have a general (systemic) effect on the human body. This helps to reduce blood flow in the veins and significantly reduce the perspiration of the liquid part of the blood through the walls of blood vessels. As a result, swelling of the mucous membrane is quickly relieved and nasal breathing is restored. The amount of nasal discharge also decreases. Eliminating swelling in the nasal cavity also improves the condition of the hearing aid.
At the same time, the drug does not have a negative effect on the mucous membrane, including its ciliated epithelium, which cleanses the nasal cavity of foreign particles. Long-term use of drops or spray (more than 5-7 days in a row) can lead to paresis of the smooth muscles of blood vessels and their prolonged dilation, accompanied by persistent swelling and nasal congestion.
The effect of the drug begins a couple of minutes immediately after it has been applied to the mucous membrane of the nasal cavity and can last for several hours.
What does Rinostop help with?
Xylometazoline, getting on the mucous membrane, narrows capillary vessels, stimulates the outflow of fluid, eliminates swelling, freeing the nasal passages and relieving congestion. This is a symptomatic drug, its compounds act locally, a small amount of the drug is absorbed into the bloodstream, which is unable to cause systemic effects for the body.
The mechanism of operation of Rinostop is based on the reaction of adrenoline receptors. The narrowing of the nasal capillaries and the reduction of swelling as a result of its use helps prevent further complicated course of the respiratory disease, prevent inflammation of the middle ear or the development of acute sinusitis.
The effect occurs 2–4 minutes after applying the solution to the mucous membranes and lasts up to 6–8 hours.
Correct use of the medicine does not lead to disruption of the functions of the ciliated epithelium and the synthesis of protective mucous secretion. The drug does not affect the general course of the disease and infectious pathogens. It reduces symptoms and restores physical comfort by restoring nasal breathing.
Indications for use of Rinostop
The drug is used:
- with nasal congestion and runny nose against the background of acute respiratory disease ;
- for chronic, including allergic rhinitis, accompanied by nasal congestion in short courses;
- for acute and chronic sinusitis ( sinusitis , sinusitis ), accompanied by nasal congestion in short courses;
- for acute and chronic otitis media to relieve swelling of the auditory tube connecting the middle ear to the nasopharynx;
- when conducting diagnostic studies (for example, rhinoscopy ) of ENT organs.
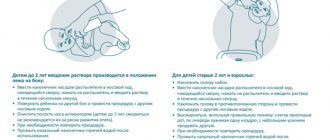
Methods and features of application
Rinostop is used by injecting a small amount of solution into the nose. Drops for adults - 1-2 in each nostril for a feeling of congestion and difficulty breathing. The frequency of use of the medicine is no more than 3 times a day. The cavities must be cleared of mucus before using the product. When placing the tip of the bottle inside, tilt your head back so that the solution does not flow out.
The spray is sprayed by carefully placing the dispenser into the nasal cavity and pressing the base of the bottle for 1 second. At the same time, you don’t have to tilt your head back. Aerosol particles penetrate directionally and are evenly distributed throughout the mucosa. You can use Rinostop spray up to 4 times a day.
It is recommended to irrigate with the solution or drip Rinostop in both new moves, even with one-sided congestion.
For children, the drug is used in the same way, choosing an age-appropriate composition. It is not advisable for children under 6 years of age to drip and spray the solution more than 2 times a day.
The maximum period of daily use of Rinostop is 7 days. If symptoms do not disappear during this time, you need to stop the course and consult a doctor to prescribe another treatment.
Erroneous actions include increasing doses or increasing the frequency of use of the solution in order to prolong its therapeutic effect. Violation of the instructions is fraught with the development of complications or the opposite of the expected effect.
Contraindications for the use of Rinostop
The medicine is contraindicated in:
- glaucoma;
- chronic atrophic (with a decrease in the volume of the mucous membrane) runny nose;
- high blood pressure;
- tachycardia;
- hypersensitivity to the components of the drug;
- while prescribing certain medications for the treatment of depression ( MAO inhibitors and tricyclic antidepressants );
- after surgery on the meninges;
- children under 2 years old.
Vasoconstrictor drops and spray should be used with caution when:
- diabetes mellitus;
- hyperthyroidism
- severe atherosclerosis , complicated by coronary heart disease ;
- prostatic hyperplasia;
- breastfeeding the baby.
Overdose
If the drug is accidentally ingested or overdose, the following symptoms may appear: anxiety, restlessness, hallucinations, convulsions, decreased body temperature, lethargy, drowsiness, coma, constriction or dilation of the pupils, fever, sweating, pallor, cyanosis, palpitations, bradycardia, arrhythmia, cardiac arrest, increased blood pressure, decreased blood pressure, nausea, vomiting, respiratory depression, respiratory arrest. Treatment: gastric lavage, taking activated carbon; symptomatic.
Side effects of Rinostop
Side effects occur mainly with overdose, long-term (more than 5 days in a row) use, or if there are contraindications for the use of this drug. , dry nose , burning , sneezing most often appear , and sometimes the runny nose worsens.
Less commonly, with long-term use, side effects manifest themselves in the form of a sharp dilation of the blood vessels of the nasal mucosa, which is accompanied by swelling of the mucous membrane, nasal congestion and heavy discharge and disturbances in nasal breathing.
In addition, with prolonged use of drops and spray without interruption, mood changes in the form of depression may occur.
Pharmacological effect
Xylometazoline belongs to alpha-adrenergic agonists - substances that stimulate the activity of specific receptors in the vascular walls that are sensitive to adrenaline and nonadrenaline. The result of its action is a rapid narrowing of the lumen and a decrease in capillary permeability upon contact with mucous membranes. Thanks to this, swelling at the site of inflammation is significantly reduced, the intensity of mucous discharge during rhinitis is reduced, and it becomes possible to breathe normally.
The vasoconstrictor effect of xylometazoline is not accompanied by disturbances in the condition of the mucosa or changes in its functions. The ciliated epithelium, which traps particles of dust and other contaminants penetrating into the nasal cavity, does not reduce activity during the use of Rinostop.
The use of the drug also does not have a systemic effect. Xylometazoline is absorbed into the general bloodstream in small quantities, narrowing only the capillaries of the mucous membrane. Rinostop does not affect the duration of diseases and the intensity of their course. Its task is to eliminate negative symptoms of congestion and restore normal well-being during illness.
The effect of using the solution occurs within 2–5 minutes. after applying it to the mucous membranes: the swelling resolves, the lumen of the nasal passages increases, opening up access to air. The effect of the drug lasts up to 5–7 hours.
Instructions for Rinostop
0.1% spray is used for adults and children over 6 years of age. The spray is sprayed after removing the cap from the bottle. The sprayer is inserted into one nasal passage, after which the base is pressed and the drug is applied for a second. Then the spray is introduced into the second nasal passage and the manipulation is repeated. You can repeat the procedure no more than 3–4 times a day. Instructions for the use of Rinostop in the form of a spray for children 2–6 years old include the administration of 0.05% spray no more than three times a day.
0.1% nasal drops are also used for adults and children over 6 years of age, 1–2 drops in each nasal passage no more than three times a day. 0.05% children's Rinostop is administered 1 - 2 drops into each nasal passage no more than twice a day.
Rinostop Spray Double Help: instructions for use
Read these instructions carefully before you start using this medicine because they contain information that is important to you. Save the instructions, you may need them again. If you have any questions, consult your doctor. The medicine you are using is intended for you personally and should not be given to others as it may cause harm to them even if they have the same symptoms as you.
Registration number: LP-005808
Trade name: Rinostop® Double Help
International nonproprietary name: Xylometazoline + [Dexpanthenol]
Dosage form: dosed nasal spray
Composition for 1 dose:
Active ingredients: xylometazoline hydrochloride – 0.1 mg, dexpanthenol – 5 mg. Excipients: sodium citrate dihydrate - 0.509 mg, sodium chloride - 0.250 mg, hypromellose - 0.100 mg, benzalkonium chloride - 0.015 mg, citric acid monohydrate - 0.350 mg, purified water up to 100 µl.
Description:
Transparent colorless solution.
Pharmacotherapeutic group: anticongestive agent
ATX code: R01AB06
Pharmacological properties
Pharmacodynamics
Xylometazoline - belongs to the group of local vasoconstrictors (decongestants) with alpha-adrenomimetic activity, causes constriction of the blood vessels of the nasal mucosa, reduces hyperemia and swelling of the mucous membrane, restores the patency of the nasal passages, facilitates nasal breathing and thereby reduces the symptoms of rhinitis. The effect of the drug usually occurs within a few minutes after its use and lasts up to 10 hours.
Dexpanthenol is a B vitamin and is a derivative of pantothenic acid. Dexpanthenol is converted in the body into pantothenic acid, which is an integral part of coenzyme A, and is involved in the processes of acetylation, carbohydrate and fat metabolism, in the synthesis of acetylcholine, corticosteroids, porphyrins; stimulates the regeneration of skin and mucous membranes, normalizes cellular metabolism, accelerates mitosis and increases the strength of collagen fibers. It has a regenerating, metabolic and mild anti-inflammatory effect.
Pharmacokinetics
Xylometazoline hydrochloride is practically not absorbed when applied topically; plasma concentrations are so low that they cannot be determined by modern analytical methods.
When applied topically, dexpanthenol is quickly absorbed by the skin and converted into pantothenic acid and binds to plasma proteins (mainly beta-globulin and albumin). Its concentration in the blood is 0.5-1 mg/l, in the blood serum - 100 μg/l. Pantothenic acid is not metabolized in the body (except for inclusion in Co-A) and is excreted unchanged.
Indications for use
- acute respiratory diseases with symptoms of rhinitis;
- acute allergic rhinitis;
- vasomotor rhinitis;
- sinusitis;
- otitis media (as part of combination therapy to reduce swelling of the nasopharyngeal mucosa);
- to facilitate rhinoscopy;
- to restore impaired nasal breathing after surgical interventions in the nasal cavity.
Contraindications
Hypersensitivity to the components of the drug, arterial hypertension, tachycardia, severe atherosclerosis, hyperthyroidism, glaucoma, atrophic rhinitis, dry rhinitis, porphyria, prostatic hyperplasia, surgical interventions on the meninges (history), simultaneous use with monoamine oxidase inhibitors (MAO) and tricyclics antidepressants, pregnancy, breastfeeding, children under 6 years of age.
Carefully
Diabetes mellitus, pheochromocytoma, diseases of the cardiovascular system (including coronary heart disease), increased sensitivity to the action of adrenergic drugs, accompanied by insomnia, dizziness, arrhythmia, tremor, increased blood pressure.
If you have one or more of the diseases and conditions listed above, you should consult your doctor before starting use.
Use during pregnancy and breastfeeding
The drug should not be used during pregnancy. The drug is contraindicated during breastfeeding.
Before use, if you are pregnant, think you might be pregnant, or are planning a pregnancy, you should consult your doctor.
Directions for use and doses
Intranasally.
For adults and children over 6 years old.
When using the nasal spray, the bottle must be kept in an upright position. Before use, it is necessary to clean the nasal passages.
During injection, you need to inhale lightly through your nose. One spray into each nasal passage up to 3-4 times a day.
Duration of therapy is 5-7 days.
Should not be used for more than 7 days. The duration of use of the drug in children is determined after consultation with a doctor.
Repeated use is possible only after a break of several days.
If after 5 days of treatment there is no improvement or the symptoms worsen or new symptoms appear, you should consult your doctor. Use the drug only according to the indications, method of administration and in the doses indicated in the instructions.
Side effect
The adverse events presented below are listed depending on the anatomical and physiological classification and frequency of occurrence. The frequency of occurrence is determined as follows: very often (with a frequency of more than 1/10), often (with a frequency of at least 1/100, but less than 1/10), infrequently (with a frequency of at least 1/1000, but less than 1/100) , rare (with a frequency of at least 1/10,000, but less than 1/1000), very rare (with a frequency of less than 1/10,000), not known (the frequency is unknown based on available data).
Nervous system disorders:
Very rare: anxiety, insomnia, headache, fatigue (drowsiness), hallucinations (mainly in children).
Cardiovascular system disorders:
Rarely: palpitations, tachycardia, increased blood pressure.
Very rare: arrhythmia.
Respiratory system disorders:
Very rare: swelling of the nasal mucosa, hypersecretion, nosebleeds.
Frequency unknown: burning and dryness of the nasal mucosa, sneezing.
Disorders of the musculoskeletal system:
Very rare: convulsions (especially in children).
Immune system disorders:
Very rare: allergic reactions (angioedema, skin rash, itching).
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Overdose
In cases of overdose or accidental oral use, the following symptoms may occur: mydriasis, nausea, vomiting, cyanosis, fever, convulsions, tachycardia, cardiac arrhythmias, vascular insufficiency, cardiac arrest, hypertension, pulmonary edema, respiratory dysfunction, hallucinations.
Patients may also experience symptoms of central nervous system depression, including drowsiness, decreased body temperature, bradycardia, shock, respiratory arrest, and coma.
Treatment
The use of activated carbon, gastric lavage, oxygen ventilation. To lower blood pressure, 5 mg of phentolamine in 0.9% sodium chloride solution is prescribed by slow intravenous administration or 100 mg of phentolamine orally. Vasoconstrictor drugs are contraindicated. If necessary, antipyretic and anticonvulsant drugs are used.
In case of overdose, consult a doctor.
Interaction with other drugs
With the simultaneous use of MAO inhibitors and tricyclic antidepressants, the systemic effect may be enhanced.
If you are using any other medications (including over-the-counter medications) at the same time, consult your doctor before use.
special instructions
Before use, it is necessary to clean the nasal passages. Avoid getting the drug into your eyes.
To avoid the spread of infection, it is necessary to use the drug individually. The duration of use of the drug in children is determined after consultation with a doctor.
Patients with long QT syndrome using xylometazoline may be at increased risk of developing serious ventricular arrhythmias.
Do not exceed the maximum duration and recommended doses when using the drug independently. If there is no improvement or if the symptoms of the disease worsen, you should consult a doctor.
Impact on the ability to drive vehicles and machinery
When using the drug in accordance with these instructions for use, the drug does not affect the ability to drive vehicles or machines, however, if adverse events occur, you should refrain from performing these types of activities.
Release form
Dosed nasal spray, 0.1 mg + 5 mg/dose. 60 doses (10 ml) or 100 doses (15 ml) in polymer bottles (high density polyethylene). The bottles are rolled with dosing pumps complete with a sprayer and a protective cap. A self-adhesive label is placed on the bottle.
Each bottle, along with a sprayer, a protective cap and instructions for use, is placed in a cardboard pack.
Storage conditions
At a temperature not higher than 25°C. Keep out of the reach of children.
Best before date
25 months. Do not use after the expiration date stated on the package.
Vacation conditions
Available without a prescription.
Marketing Authorization Holder/Complaint Receiving Entity
OTCPharm JSC, Russia, 123112, Moscow, st. Testovskaya, 10, floor 12, room II, room. 29, Tel. Fax www.otcpharm.ru
, Russia, 305022, Kursk, st. 2nd Aggregatnaya, 1a/18, tel./fax www.pharmstd.ru
Rinostop's analogs
Level 4 ATX code matches:
Xymelin Eco
Xymelin
Nazivin
Galazolin
Lazorin
Nazivin Sensitive for children
Otrivin
Naphthyzin
Sanorin
Knoxprey
For the nose
Lazolvan Rino
Afrin
Rhinorus
Eucazoline Aqua
Rinazolin
Grippostad Reno
Farmazolin
Xylometazoline
Nazol Advance
Analogues are drugs from different drug groups that are used to treat the same diseases. Analogues of Rinostop - drugs that relieve swelling of the nasal mucosa: naphazoline ( Naphthyzin , Sanorin ), Oxymetazoline ( Nazivin , Nazol , Knox-spray ), phenylephrine ( Nazol Baby ), tetrizoline ( Tetrin ), tramazolin ( Lazolvan Rino ), dimethindene + phenylephrine.
Side effects
Burning or dryness of the mucous membranes of the nasal cavity, dryness of the mucous membranes of the mouth and throat; sneezing; an increase in the volume of secretions released from the nose; nose bleed; after the effect of the drug wears off, a feeling of nasal congestion (reactive hyperemia). Side effects caused by the systemic effect of the drug (if the solution is accidentally taken orally): increased blood pressure, headache, dizziness, palpitations, tachycardia, restlessness, anxiety, fatigue, drowsiness, sedation, irritability, sleep disturbance (in children), nausea , insomnia, exanthema, hallucinations, Quincke's edema, itching, convulsions, respiratory arrest (in
babies). Visual impairment (if in contact with eyes). Long-term continuous use of vasoconstrictor drugs can lead to tachyphylaxis, atrophy of the nasal mucosa and recurrent swelling of the nasal mucosa (rhinitis medicamentosa). If any of the side effects indicated in the instructions or other side effects not listed in the instructions occur, consult your doctor.
Rinostop price
Rinostop price in Moscow:
- Rinostop nasal drops 0.1% and 0.05% 10 ml - 21 - 25 rubles;
- nasal spray 0.1% And 0.05% 15 ml - 56 - 65 rubles.
Rinostop tablets, produced by the pharmaceutical company Bosnalek (Bosnia and Herzegovina) are not synonymous with Rinostop drops and spray, as they have a different composition.
- Online pharmacies in RussiaRussia
ZdravCity
- Rinostop Extra 0.05% 15 mlPharmstandard-Leksredstva OJSC
RUB 147 order - Rinostop Double relief for runny nose 6+, nasal spray + xylometazoline, 15 mlPharmstandard-Leksredstva OJSC
RUB 154 order
- Rinostop Aqua Baby for children 0+ for runny nose, nasal spray, 100% sea water 125 mlLaboratory De La Mer
RUB 339 order
- Rinostop 0.05% 15 mlPharmstandard-Leksredstva OJSC
95 rub. order
- Rinostop 0.1% 15 mlPharmstandard-Leksredstva OJSC
96 RUR order