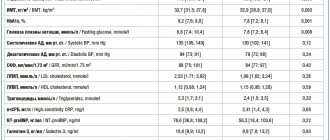
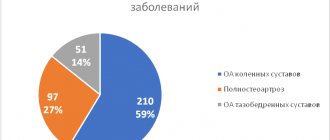
Pembrolizumab (Keytruda, Keytruda, MK-3475) is an innovative drug that was approved by the FDA (American Food and Drug Administration) in September 2014, and is currently widely used for the treatment of late-stage melanoma.
In 2014, Keytruda received Priority Review and Breakthrough Medical Designation. This means that Pembrolizumab has been included in a group of medicines that can improve the effectiveness and safety of the treatment of rare and serious diseases. This is why the drug was approved and introduced into clinical practice so quickly - it usually takes much longer.
How does Pembrolizumab work?
Drugs for immunotherapy of melanoma and other malignant tumors have been around for quite some time. But they have always had low efficiency, and until recently scientists did not know how to deal with this problem.
The goal of immunotherapy is to activate the patient’s immune system, causing it to attack and destroy cancer cells. For a long time, the PD-1 protein became an obstacle to achieving this goal.
The PD-1 protein blocks the immune system. It prevents T lymphocytes from recognizing and destroying cancer cells. Keytruda contains monoclonal antibodies that block PD-1. The drug helps remove the “brake”, due to which lymphocytes acquire the ability to attack tumor tissue.
Release form
Concentrate for the preparation of solution for infusion. Transparent or opalescent solution from colorless to light yellow.
1 bottle contains:
active ingredient: pembrolizumab 100.0 mg;
excipients: L-histidine 1.2 mg; L-histidine hydrochloride monohydrate 6.8 mg; polysorbate – 80 0.8 mg; sucrose 280 mg; water for injection up to 4.0 ml.
4 ml of the drug in a type I flint glass bottle (European Pharm., US Pharm.), sealed with a chlorobutyl rubber stopper, crimped with an aluminum cap and protected with a plastic cap.
How effective is Pembrolizumab?
A study was conducted at the University of California, Los Angeles, which involved 173 people diagnosed with advanced melanoma. They were divided into two groups. In one of them, patients received a standard dose of the drug 2 mg per kilogram of body weight every 3 weeks. In the second group, the dose was increased 5 times (10 mg/kg).
In 24% of patients receiving the drug at a dose of 2 mg/kg, the tumor decreased by more than a third. Re-growth of melanoma was not observed, and the effect of the drug lasted from 1.4 to 8.5 months (in some cases longer).
MSD reported that pembrolizumab in combination with chemotherapy (CT) demonstrated improvements in overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) in patients with advanced non-small cell lung cancer (NSCLC) without PD-L1 expression in tumors (TPS < 1%) when used in the first line of therapy.
According to a pooled analysis of the KEYNOTE-189, KEYNOTE-407 and KEYNOTE-021 studies (Cohort G), pembrolizumab in combination with chemotherapy provided a 44% reduction in the risk of death (RR = 0.56) compared with chemotherapy. In the pembrolizumab + chemotherapy group, the ORR was 46.9%, compared with 28.6% in the chemotherapy group. The safety profile of pembrolizumab was consistent with previous clinical trials in patients with advanced NSCLC.
"The goal of our lung cancer clinical research program is to find ways to improve the survival rate of patients diagnosed with the disease," said Dr. Jonathan Cheng, vice president of MSD's oncology clinical research division. “This analysis confirms the effectiveness of pembrolizumab in combination with chemotherapy in patients with NSCLC who do not have PD-L1 expression in the tumor (TPS<1%).”
“Based on the overall survival benefit demonstrated in clinical trials, pembrolizumab in combination with chemotherapy has become the standard first-line treatment for patients with advanced non-small cell lung cancer in the absence of activating mutations,” said Dr. Hossein Borghaei, Chief Executive Officer of thoracic oncology department.
Additional data from a pooled subgroup analysis of KEYNOTE-189, KEYNOTE-407 and KEYNOTE-021 (Cohort G)
Data presented during the World Conference on Lung Cancer come from a pooled subgroup analysis of 428 treatment-naïve patients without tumor PD-L1 expression (TPS < 1%) who participated in the KEYNOTE-189 (non-squamous NSCLC) trials. ; n = 190), KEYNOTE-407 (squamous NSCLC; n = 194), and KEYNOTE-021 (non-squamous NSCLC; n = 44 [Cohort G]). Patients were randomized to receive pembrolizumab 200 mg every 3 weeks plus chemotherapy (n = 243) or chemotherapy (n = 185). Patients with non-squamous NSCLC received treatment with pemetrexed in combination with a platinum drug, patients with squamous NSCLC received carboplatin in combination with paclitaxel or nab-paclitaxel. Patients with genetic aberrations of EGFR or ALK were not allowed to participate in the studies. Key efficacy objectives included OS, PFS, and ORR.
Pembrolizumab in combination with chemotherapy demonstrated a 44% reduction in the risk of death (RR = 0.56 [95% CI, 0.43–0.73]) over a median follow-up of 10.2 months compared with chemotherapy. Estimated overall survival rates at 12 months in the pembrolizumab plus chemotherapy group were 66%, compared with 47% in the chemotherapy group; overall survival rates at 18 months were 52% and 29%, respectively. Median OS in the pembrolizumab plus chemotherapy group was 19.0 months (95% CI, 15.2–24.0), compared with 11.0 months (95% CI, 9.2–13.5) in the chemotherapy group .
The combination of pembrolizumab with chemotherapy also reduced the risk of disease progression or death by 33% (RR = 0.67 [95% CI, 0.54–0.84]) compared with chemotherapy. Estimated progression-free survival rates at 12 months in the pembrolizumab plus chemotherapy group were 29%, compared with 17% in the chemotherapy group; progression-free survival rates at 18 months were 22% and 9%, respectively. Median PFS in the pembrolizumab plus chemotherapy group was 6.5 months (95% CI, 6.2–8.5), compared with 5.4 months (95% CI, 4.7–6.2) in the chemotherapy group .
The ORR in the pembrolizumab plus chemotherapy group was 46.9% (n = 114) and in the chemotherapy group the ORR was 28.6% (n = 53). The median duration of response in the pembrolizumab plus chemotherapy group was 7.9 months (range, 1.1+ to 28.4+), and in the chemotherapy group this figure was 6.7 months (range, 1.4+ to 30). ,1+). 42.4% of patients in the pembrolizumab plus chemotherapy group had a response duration of 12 months or more, compared with 35.3% in the chemotherapy group.
Among patients in the pembrolizumab plus chemotherapy group, the rate of grade 3–5 adverse events (AEs) was 68% (n = 165), compared with 72% (n = 131) in the chemotherapy group. The incidence of grade 3–5 AEs leading to death was 9% (n = 23) in the pembrolizumab plus chemotherapy group and 6% (n = 11) in the chemotherapy group. The incidence of grade 3–5 immune-related AEs and infusion-related reactions in the pembrolizumab plus chemotherapy group was 11% (n = 27), compared with 3% (n = 5) in the chemotherapy group. Fatal outcomes due to immune-mediated AEs and infusion-related reactions were observed in 1% of cases (n = 2) in the pembrolizumab plus chemotherapy group, whereas there were no deaths in the chemotherapy group.
About pembrolizumab
Pembrolizumab is a humanized monoclonal antibody that selectively blocks the interaction between the PD-1 receptor on immune T cells and the PD-L1 and PD-L2 ligands on tumor cells.
Pembrolizumab was first approved in 2014 in the United States for the treatment of patients with unresectable and metastatic melanoma whose disease has progressed after prior therapy. The drug was later approved for the treatment of patients for 14 indications.
Pembrolizumab was first registered in Russia in November 2016. The drug is currently approved for use in nine indications for the treatment of patients:
Melanoma
- for the treatment of adult patients with inoperable or metastatic melanoma;
- as adjuvant therapy in patients with melanoma with lymph node involvement after surgical treatment.
Non-small cell lung cancer
- in combination with chemotherapy, including a platinum drug and pemetrexed as 1st line of therapy in patients with metastatic non-squamous non-small cell lung cancer in the absence of mutations in the epidermal growth factor (EGFR) or anaplastic lymphoma kinase (ALK) genes;
- in combination with carboplatin and paclitaxel or albumin-stabilized nanodispersed paclitaxel as 1st line of therapy in patients with metastatic squamous non-small cell lung cancer;
- as 1st-line monotherapy in patients with locally advanced or metastatic non-small cell lung cancer with PD-L1 expression ≥ 1% by tumor cells, determined by a validated test, in the absence of mutations in the EGFR or ALK genes;
- as monotherapy for the treatment of patients with advanced non-small cell lung cancer with PD-L1 expression ≥ 1% by tumor cells, determined by a validated test, who have previously received platinum-containing therapy. If there are mutations in the EGFR or ALK genes, patients must receive appropriate specific therapy before they are prescribed treatment with the drug.
Head and neck cancer
- for the treatment of patients with recurrent or metastatic squamous cell carcinoma of the head and neck whose disease has progressed during or after platinum-containing chemotherapy.
Classic Hodgkin lymphoma (cHL)
- for the treatment of adults and children with refractory classical Hodgkin lymphoma or with relapse of the disease after three or more previous lines of therapy.
Urothelial carcinoma
- for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are unable to receive cisplatin-containing chemotherapy with PD-L1 expression (combined positive score (CPS≥ 10) according to a validated test, as well as in patients who cannot chemotherapy with any platinum drugs, regardless of PD-L1 expression;
- for the treatment of patients with locally advanced or metastatic urothelial carcinoma who have previously received platinum-containing chemotherapy
Stomach cancer
- for the treatment of patients with recurrent locally advanced or metastatic adenocarcinoma of the stomach or esophagogastric junction with PD-L1 expression (combined positive score (CPS≥ 1) according to a validated test. Patients should have documented disease progression during or after treatment two or more lines of previous therapy, including chemotherapy with fluoropyrimidines and platinum drugs, as well as, if necessary, targeted therapy with anti-HER2/neu drugs.
Malignant neoplasms with a high level of microsatellite instability
- for the treatment of patients with advanced malignancies with a high level of microsatellite instability (MSI-H), including disorders of the DNA repair system (dMMR), who have previously received therapy
Hepatocellular carcinoma
- for the treatment of patients with hepatocellular carcinoma (HCC) who have previously received anti-angiogenic therapy with tyrosine kinase inhibitors (TKIs).
Cervical cancer
- for the treatment of patients with recurrent or metastatic cervical cancer with PD-L1 expression (CPS≥ 1) according to a validated test with disease progression during or after chemotherapy.
About immuno-oncology
Recent discoveries in the field of immuno-oncology open up new opportunities for patients with malignant neoplasms in treating their diseases and increasing life expectancy. Immuno-oncology drugs enhance the immune system's natural ability to fight tumors. Unlike chemotherapy (which inhibits cell division of rapidly growing tumor cells) and targeted therapy (which affects various molecular targets on tumor cells), immuno-oncology drugs target various components of the immune system, including immune checkpoints that are normally responsible for regulating the immune system. .
About MSD
For more than 125 years, MSD has been one of the leading global healthcare companies. MSD is a brand name of Merck & Co. Inc. is headquartered in Kenilworth, New Jersey, USA.
We discover, develop and manufacture innovative prescription medicines, including biologics and vaccines, that help maintain and improve people's health. The MSD portfolio includes drugs for the prevention and treatment of cancer, diabetes mellitus, hepatitis C, HIV infections, autoimmune inflammatory and respiratory diseases, diseases of the circulatory system and other nosologies.
We implement and support programs and partnerships that improve the quality of medical care.
MSD has been operating in Russia since 1991, focusing on ensuring the availability of innovative medicines and vaccines, partnering with local manufacturers and leading medical institutions, and supporting medical education. We apply our wealth of international experience to contribute to the development of the Russian healthcare and pharmaceutical industry.
Is Keytruda safe?
The second study was conducted on 411 patients who had advanced melanoma and were taking Keytruda. However, severe side effects from the intestines, lungs and liver were rarely observed.
Most often, patients experienced side effects such as increased fatigue, cough, nausea, rash, itching, loss of appetite, constipation, diarrhea, and joint pain (source).
The information is for reference only. Euroonko cooperates with Israeli, European and American doctors who have accumulated significant experience with the drug Keytruda.
Book a consultation 24 hours a day
+7+7+78
KEYNOTE-185 Study
The KEYNOTE-185 (phase 3) trial assessed the efficacy and safety of lenalidomide in combination with low-dose dexamethasone with or without pembrolizumab in patients with multiple myeloma who are not eligible for autologous stem cell transplantation.
- Of 301 randomized patients, 19 died in the pembrolizumab group, compared with 9 patients in the control group (hazard ratio, 2.06 (95% CI: 0.93-4.55)).
- There was a 22% increased risk of severe toxicity (grade 3-5) in the pembrolizumab group, as well as a higher incidence of serious adverse events (54% vs. 39%).
- The causes of death in patients from the pembrolizumab group, not associated with disease progression, were pulmonary embolism, intestinal ischemia, myocarditis, heart failure, sudden death, pneumonia.
Source:
FDA. Drug Safety and Availability. August 31, 2017
Interaction with other drugs
Specific studies of the pharmacokinetic interaction of Keytruda with other drugs have not been conducted. Since pembrolizumab is eliminated from the circulation by catabolism, metabolic drug interactions should not be expected.
The use of systemic corticosteroids or immunosuppressants should be avoided before initiating Keytruda therapy, given their possible effect on the pharmacodynamic activity and efficacy of Keytruda. However, systemic corticosteroids or other immunosuppressants may be used after initiation of pembrolizumab treatment for the treatment of immune-mediated adverse reactions (see Precautions).